SN1 Full Form
What is the full form of SN1?
SN1 is a reaction mechanism in organic chemistry. SN1 stands for “Substitution Nucleophilic Unimolecular”. It is a multi-step process in which a carbocation is formed due to a leaving group getting removed. Then the attack of a nucleophile takes place which results in the formation of a new carbon compound. The rate of the reaction depends only on the electrophilicity of the group leaving.
More about the SN1 reaction
The SN1 reaction is a substitution reaction where the step that determines the rate of the complete reaction is unimolecular. By dependence on the electrophilicity of the leaving group, it means that the nucleophile intermediate is much more stable than the carbocation intermediate.
This reaction is generally observed in secondary or tertiary alkyl halides with tertiary or secondary alcohols. The condition for the reaction to take place must be strongly acidic or strongly basic. In inorganic chemistry, this reaction is referred to as a dissociative mechanism.
Example-
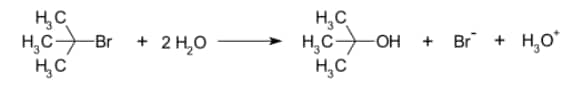
Effect of Solvent
If the solvent favours the formation of carbocation then the rate of reaction increases. Both polar and protic solvents are preferred for this reaction. Ionic intermediates stabilise quickly in polar solvent whereas the leaving group gets solvated in the protic solvent. Some examples of solvents that are used in SN1 reactions are water and alcohol.
Reaction Mechanism
Let us see the mechanism of SN1 with the help of an example. Here we will see the hydrolysis of tertiary butyl bromide.
In the first step, the formation of Tertiary butyl carbocation takes place. Tertiary butyl carbocation is formed by the bromide ion leaves.
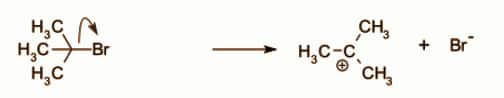
Since this is the slowest step, it is the rate-determining step of the overall reaction. Now the carbocation is formed.
The next step is called the Nucleophilic attack. Here the OH group of water acts as a nucleophile. The intermediate ion formed from water is called an oxonium ion. This step of the reaction is fast.
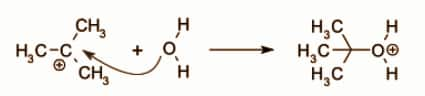
The last step of the reaction is Deprotonation. The nucleophile has an extra plus. This removal is done with the help of water which results in the formation of alcohol and a hydronium ion.

Frequently Asked Questions (FAQs)
In SN1 reactions, nucleophiles are not a part of the rate-determining step which means the strength of nucleophiles does not matter. Since carbon becomes a carbocation, it looks for an electron donor and immediately makes a bond with it.
The affinity of an ion, atom or molecule to accept an electron is known as its electrophilicity.
A substitution reaction is a reaction in which a group of higher power substitutes a group that has lower power, from its compound.
Carbocation is a molecule of carbon which has 3 of its bonds formed and possesses a positive charge. Earlier, it was termed a carbonium ion.
When a species forms a bond with another species by donating the electron pair, then those species are called nucleophiles.