SPDF Full Form
What is the full form of SPDF?
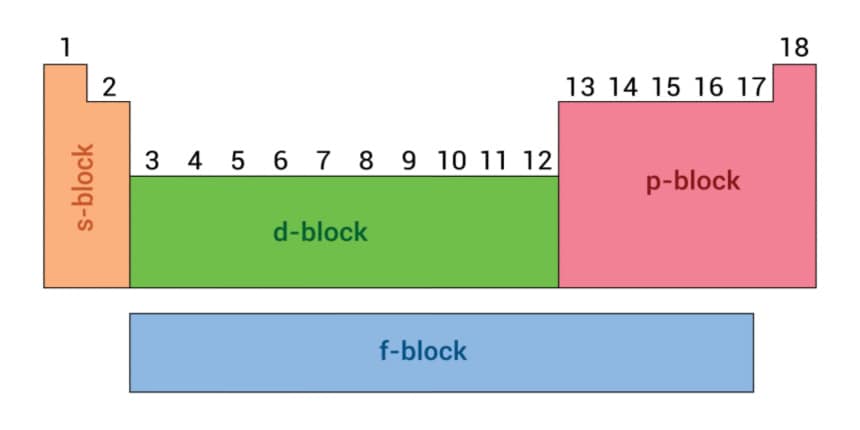
In chemistry, s,p,d, and f refers to the orbitals. Orbitals are the region or spaces where the probability of finding an electron is not zero. Each of the orbitals is denoted by a number or letter to identify the electron’s energy levels. The name of the orbital, i.e. s,p,d and f, are given according to the names given to the groups of the line originally noted in the spectra of alkali metals. Each atomic orbital is associated with three quantum numbers, i.e. n,l and m.
The principal quantum number denoted by "n" tells the energy and size of the orbital. The Azimuthal quantum number denoted by "l" represents the orbital three-dimensional shape. The magnetic quantum number or "m" tells the orbitals’ spatial orientation. Also, the electron spin or "s" value tells the spin of electrons in each orbital but has no classical analogue.
Types Of Orbital:
s-orbital-
It is a spherical-shaped and symmetrical orbital.
Its energy level increases as we move away from the nucleus.
The probability of finding an electron decreases with an increase in energy level i.e. probability is maximum in 1s and decreases in 2s.
A point is found when there is zero probability of finding an electron. It is known as a nodal point.
The nodal point is further divided into two i.e.radial nodes that calculate distance from the nucleus and angular nodes that determine direction.
Angular node is equal to "l", i.e. Azimuthal quantum number.
Radial nodes are given by "n-l-1".
p-orbital-
It is a dumbbell-shaped orbital whose nodes are at the centre of the nucleus.
It can occupy six electrons in its orbital.
Each p-orbital has lobes on either side of the plane passing through the nucleus.
These lobes are oriented along the x,y and z axes and thus named according to them. The “dxy” orbital has 2 nodal planes passing through the origin.
d-orbital-
The shape of the d-orbital is a double dumbbell shape or clove leaf shape.
There are five d-orbitals that are equal in energy and thus can accommodate 10 electrons in it.
f-orbital-
The shape of the f-orbital is diffused.
It has seven orbitals and thus can accommodate 14 electrons in it.