Discovery of Electron
Discovery of electron
Two of the founding laws of chemical sciences given by Lavoisier i.e., (laws of chemical combinations, and law of constant proportion) led to upliftment in the discussions on the basic unit of matter from philosophy to science. Dalton who was a school teacher came forward and selected the Greek name atom as the basic unit of matter. He laid a few points to explain the above laws in his theory which is now known as “Dalton’s atomic theory”.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
However, the indivisibility of an atom remained a highly controversial point which was also proved wrong later after some of the very famous experiments of the times. Around the twentieth century, many experiments led to the discovery of sub-atomic particles.
Founder of electron
J.J Thomson invented an electron in his model of an atom where he compared the structure of the atom to the Christmas pudding.
- The position of the electron was fixed inside the sphere just like the dry fruits in the pudding. Or think of watermelon. The black seeds according to him were like the electrons and the red edible fibrous part of the watermelon is like the positive charge spread throughout the sphere.
- He proved the neutrality of the atom by pointing out the fact that protons and electrons of the atom are equal in magnitude.
His theory, like Dalton’s, also came out as faulty and incomplete after a few experiments conducted in the future. J.J Thomson received Nobel Prize in 1906 for his work on the discovery of electrons.
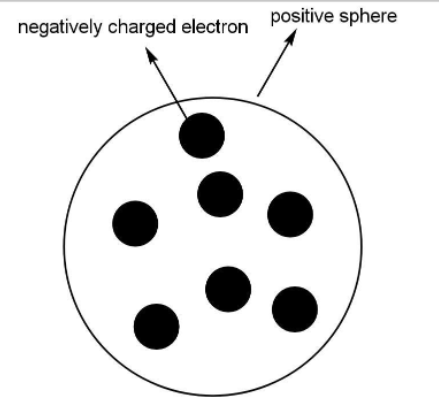 Thomson's model of an atom
Thomson's model of an atom
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Discovery of electron
Electron invented by J.J Thomson was one of the most important discoveries in the field of chemistry.
Our journey inside the atom started after Michael Faraday pinpointed the relationship of matter with electricity.
1n 1874, Stoney pointed that electricity is made up of discrete units of electricity.
Discharge tube experiments
- When electricity is passed through a gas at low pressure inside a glass tube such kinds of experiments are known as discharge tube experiments. These experiments can tell the nature of sub-atomic particles.
- These experiments have to be carried at a higher voltage because, at a lower voltage, gases behave as poor conductors of electricity.
- J.J Thomson was given the credit to produce electricity through gases under high voltages.
Electricity flow was observed in the form of rays and these rays were called cathode rays.
- The study of these cathode rays led to the discovery of electrons.
Related Topics, |
Apparatus for the experiment
1)A cylindrical hard glass tube that is closed from both ends is required, this tube is called a discharge tube.
2)Two electrodes made of metal are fitted inside.
3)A side tube at the side is required through which pressure can be set by evacuation.
4)A vacuum pump.
5)The gas taken for the experiment is filled inside the cathode ray tube.
6)And a high voltage.
 Discharge tube
Discharge tube
Observations of the experiment
- At normal pressure, no observations are made because the gas under the study is poor conductor of electricity. Even at voltages of the order (5,000-10,000 V), no observations are made.
- With the help of a vacuum pump, the pressure of the gas is reduced by pumping the gas out.
A decrease of 10-2 atm is made to make the gas conducting. - This conducting gas then gives a signal by the light of different colors. The color imparted will depend upon the gas taken inside the tube.
- However, a further decrease in the pressure will only lead to a weaker glow of the gas. At one point this glow is completely stopped but the conducting nature of the gas remains.
- At this point anode side of the tube starts to glow up and the color noticed is greenish light.
Observation of the experiment-
- It was concluded that cathode rays were being emitted by the cathode end of the tube. The glow was due to the bombardment of the glass with these rays.
- A hole was drilled in the anode to confirm the direction of the movement of these rays. The backside of this hole was coated with zinc sulfide. Zinc sulfide is a fluorescent material so that every time these rays would travel from cathode to anode, a bright spot will emerge on the anode. These cathode rays moved in a straight line from the cathode end of the tube to the anode end of the tube.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 2 Structure of Atom
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 2 Structure of Atom
- NCERT notes Class 11 Chemistry Chapter 2 Structure of Atom
Conclusion of the experiment
Many conclusions were drawn by conducting a few experiments to understand the nature of these cathode rays. Inventor of electron used, application of magnetic field and electric field to confirm the nature of cathode rays.
- If an object was kept between the path of cathode rays the casting of shadow will help in the origination of cathode rays. The position of shadow was bent opposite to the wall of the cathode. Flourosense was observed in the region outside the shadow. This shows cathode rays travel in a straight line and these rays travel from cathode to anode.
- Since these rays are not visible but these can still be sensed through a fluorescent material that glows up when struck upon.
- If a wheel is placed between the electrodes it starts rotating. Meaning that cathode rays show a mechanical effect.
- In the absence of a magnetic field and electric field, these rays travel perfectly in a straight line but with the introduction of such a field, these rays show a deflection. This observation shows that cathode rays consist of particles that are charged in nature.
- Effect of electric field-To study the effect of electric field the arrangement was modified a bit by the addition of metal plates above and below the tube. When these plates are oppositely charged, cathode rays are preferentially bent to the positively charged plate. This means that the particles of the cathode must be negatively charged.
- Effect of magnetic field- In the presence of a magnetic field the cathode rays are deflected in the direction which corresponds to negatively charged particles.
Cathode rays produce a heating effect when struck against a metal foil. Cathode rays also produce X-rays against hard metals.
Charge and mass of an electron- the magnitude of the electron were calculated based on the principle that the greater is the charge on the electron greater will be the interaction of those rays with the magnetic and electric field. the nature and properties of cathode rays are independent of the gas taken in the tube.
The charge to mass ratio is the same for every gas.
- The mass of particles depends upon the extent of deviation of that particle. If the mass of the particle will be lighter then its deflection will be greater.
- Thomas calculated the mass of electrons by collecting the data from the deflection of the particle.
Electron characteristics
Electrons are discovered by J.J Thomson.
- Mass of electron as compared to atom is much smaller.
Therefore, an electron can be defined as the subatomic particle of an atom carrying a unit negative charge (1.6022 × 10-31C) and the mass of this particle is equal to 9.1 ×10-31kg.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
J.J Thomson, a high school teacher discovered electrons in the late 19th century.
Electron is a negatively charged particle carrying a charge of -1.6022 × 10-31C and a a mass of 9.1 × 10-31C kg.
The relative charge of an electron is -1.and the relative mass is 5.4858 ×10-4.
Proton is a positively charged particle carrying a charge of +1.6022 × 10-31C and a mass of 1.67262 ×10-27 kg.
The relative charge of the proton is +1 and the relative mass is 1.67262 ×10-27
A neutron is a neutral particle carrying a charge of zero. and a mass of 1.67262 ×10-27kg.
The relative charge of the neutron is zero and the relative mass is 1.67493 ×10-27kg.
G. Johnstone Stoney coined the term electron after figured the relationship between electricity and matter.
J.J Thomson
Electron was discovered after the discovery of cathode rays which were observed to travel from a straight line and the effect of electric field and magnetic field agreed with the nature of the electron.
Also Read
06 Feb'25 11:21 PM
30 Dec'24 03:07 PM
20 Dec'24 11:57 PM
16 Dec'24 11:39 PM
16 Dec'24 11:27 PM
16 Dec'24 11:16 PM
21 Oct'24 04:01 PM
21 Oct'24 12:25 PM