Formation of Ionic Compounds - Structure, Properties, Examples, FAQs
How are ionic compounds formed?
What happens when metals and non-metals react? The two molecules form ionic bonds, which are then used as building blocks. Ionic bonds are electrostatic forces of attraction between two oppositely charged atoms. Whenever an electron is transferred from a metal to a non-metal, an ionic bond is formed. The cation and anion form an ionic bond. Positive ions have an anion charge, while negative ions have a cation charge.
Define ionic compounds and write the properties of ionic compounds?
Ionic compound definition: A unique atom can only be determined by its particular number of protons, neutrons, and electrons. The number of protons and electrons in an atom is typically equal to one. Because it would mean creating an entirely different element, the protons in an atom would never change. It should be noted, however, that atomic electron numbers fluctuate.
Read more :
- NCERT notes Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure
An atom can have an ion when its electron is gained or lost. Adding or removing electrons to an atom will alter its charge because electrons have a negative charge already. The electron number becomes out of phase with the positively charged protons.
The net negative charge gained by an atom is caused by anions, which gain an electron. A net positive charge is obtained by losing electrons; a process known as cationization. Cations are metals, whereas anions are nonmetals. It is also possible to refer to an ion as a single atom or a complex group of atoms.
The opposites do indeed attract when it comes to ions. It is formed by ionic compounds when the opposite charges, negative and positive, hold together. The compound is made up of ions, as its name implies. Each atom is lost or gained at the same rate. A pair of electrons forms when the atom that needs it donates an electron to the atom that needs it.
Ions can be compared to two bars of a magnet. Magnets that have the same magnetic field repel each other when the ends are put together. Putting the north end of a magnet to a sound end makes it snap together, but turning it around makes it stay apart. It is similar for ions to form ionic compounds when two positive and two negative ions combine together.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Properties of Ionic Compound
The characteristics of ionic compounds are
- Ionic compounds are solid and not easily breakable due to the strong force of attraction between positive and negative ions. They are brittle because they break into pieces when pressure is applied.
- Boiling and melting points: Ionic compounds have a strong force of attraction between each other. Therefore, breaking the ionic bonds between atoms requires a lot of energy. The melting and boiling points of ionic compounds are higher because of this.
- It is possible for ionic compounds to dissolve in polar solvents. Solvents with a polar configuration are methanol, water, and formamide. Nonpolar solvents like chloroform, hydrocarbons, etc. have little or no effect on ionic compounds.
- A solid form of an ionic compound does not conduct electricity. However, they are conducted in molten states. Conducting electricity involves transferring a charge from one place to another.
Ionic compound Structure
The relative size of the cations and anions determines the structure of an ionic compound. A variety of inorganic and organic compounds contain ionic charges, such as salts, oxides, sulphides, hydroxides, etc. Positive and negative ions attract each other electrostatically, which holds ionic solids together.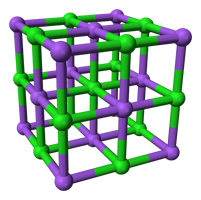
Related Topics Link |
Sodium Chloride
It is possible to have a 3-D structure for sodium ions and chloride ions when sodium ion attracts chloride ion and vice versa. Sodium chloride crystals are all it is. A substance that contains equal amounts of sodium ions and chloride ions doesn't have any charge. In order for the structure to remain in place, ions have to attract one another.
NCERT Chemistry Notes:
Ionic Solid Examples or Example of Ionic Solid:
A magnesium-chlorine reaction, for ionic bond examples. Two electrons are located in the outermost shell of the magnesium atom. A molecule's L shell becomes the outermost shell that has a stable octet when its M shell loses two electrons. The nucleus of this magnesium atom has as many protons as before, but it has lost ten electrons. A net positive charge is generated on this magnesium atom, which gives rise to a magnesium cation, Mg2+.
The chlorine atom, however, has seven electrons, which are located in its outermost shell. Thus, its octet can be completed by adding a single electron. The magnesium atom can gain this one electron by gaining electrons it lost as it splits into two magnesium ions. Two chlorine atoms can gain one electron, while one magnesium atom can lose two electrons, so two chlorine atoms bind with one magnesium atom to form magnesium chloride.
By using the examples of the ionic compound above, ionic compounds can be defined as compounds produced when electrons are transferred between metals and nonmetals. They form an ionic bond between each other. Ionic compounds are strongly attracted by electrostatic forces because they contain oppositely charged ions
Also, check-
