2 4 Dinitrophenylhydrazine - Structure, Preparation, Brady’s Reagent, FAQs
Have you ever wondered how we can detect and identify carbonyl compounds like aldehydes and ketones in a sample? What role does 2,4-dinitrophenylhydrazine play in this process? How does it react with carbonyl compounds to form a dinitrophenylhydrazone derivative, making the identification easier? 2,4-dinitrophenylhydrazine is generally abbreviated as 2,4-DNP, also known as Brady’s reagent. This is generally present in solid form and orange-red in color. It is very reactive in nature, so to avoid the risk of explosion, we keep it in water, or we can say it is stable in wet conditions as compared to dry ones.
This Story also Contains
- 2,4-Dinitrophenylhydrazine Structure
- Preparation of 2,4-Dinitrophenylhydrazine From Chlorobenzene
- Mechanism Of Synthesis Of 2,4-DNP
- Brady’s Reagent
- 2,4-Diphenylhydrazine Laboratory Test
- Reaction of 2,4-DNP With Ethanal
- Some Solved Examples
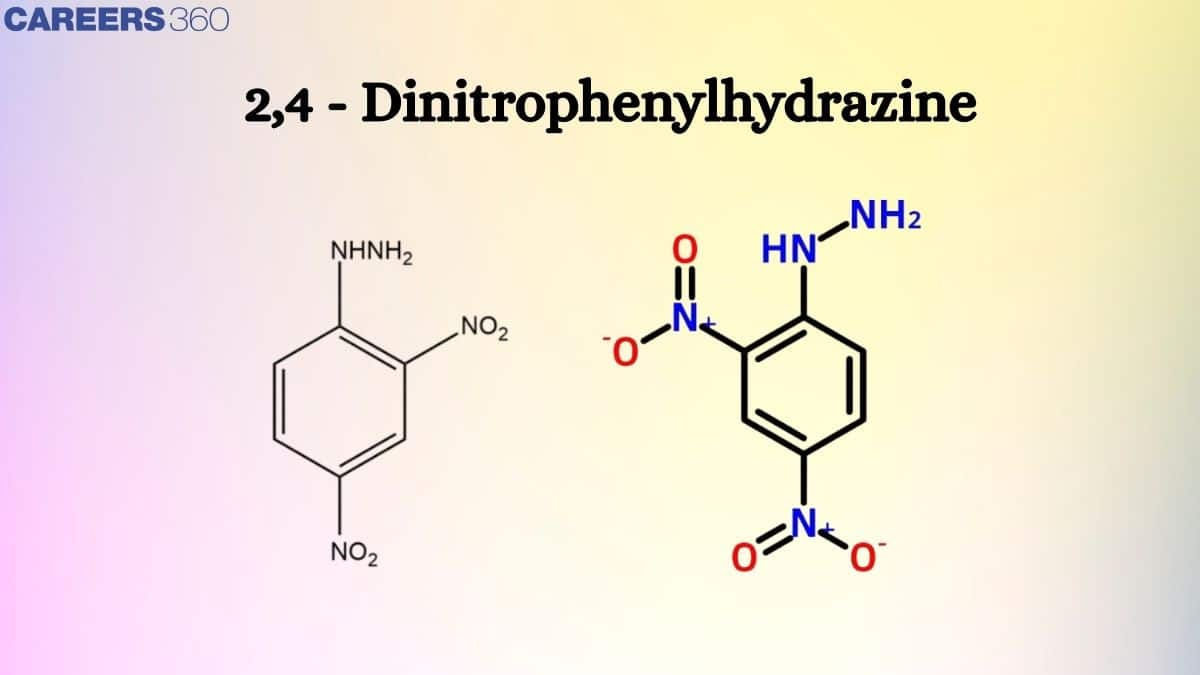
2,4-Dinitrophenylhydrazine Structure
2,4-DNP structure can be shown as follows, where DNP full form is dinitrophenylhydrazine
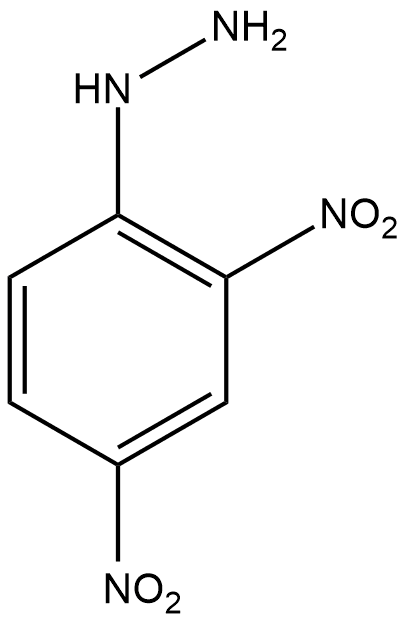
As the name suggests, 2,4-dinitro, i.e. presence of two nitrogen groups and phenyl hydrazine is represented by a benzene ring with $\mathrm{NH}-\mathrm{NH}_2$This group is called phenyl hydrazine.
The main use of 2,4-DNP and its derivatives gives us a method of separating the components of compounds containing aldehydes and ketones. Aldehydes and ketones can easily be separated out with the help of fractional distillation, but we can easily separate 2,4-DNP and its derivatives with the help of column chromatography. One more advantage of this is that formation from aldehydes and ketones is reversible in nature, and separated derivatives will be regenerated easily to their original carbonyl compounds with the help of hydrolysis of those derivatives.
Also read -
Preparation of 2,4-Dinitrophenylhydrazine From Chlorobenzene
2,4-DNP is not said to be a commonly known substituted hydrazine, but it can be prepared with the help of resonating phenomenon. With this, we can easily make a reasonable inference which suggests that hydrazine, represented by the molecular formula $\mathrm{NH}_2-\mathrm{NH}_2$, acts as a good nucleophile. We can prepare the 2,4-DNP with the reaction of hydrazine with 2,4-dinitrochlorobenzene. The presence of two nitro groups makes the compound available for electron-accepting, which makes it easy to displace these two chloride ions. The reaction can be shown as follows:
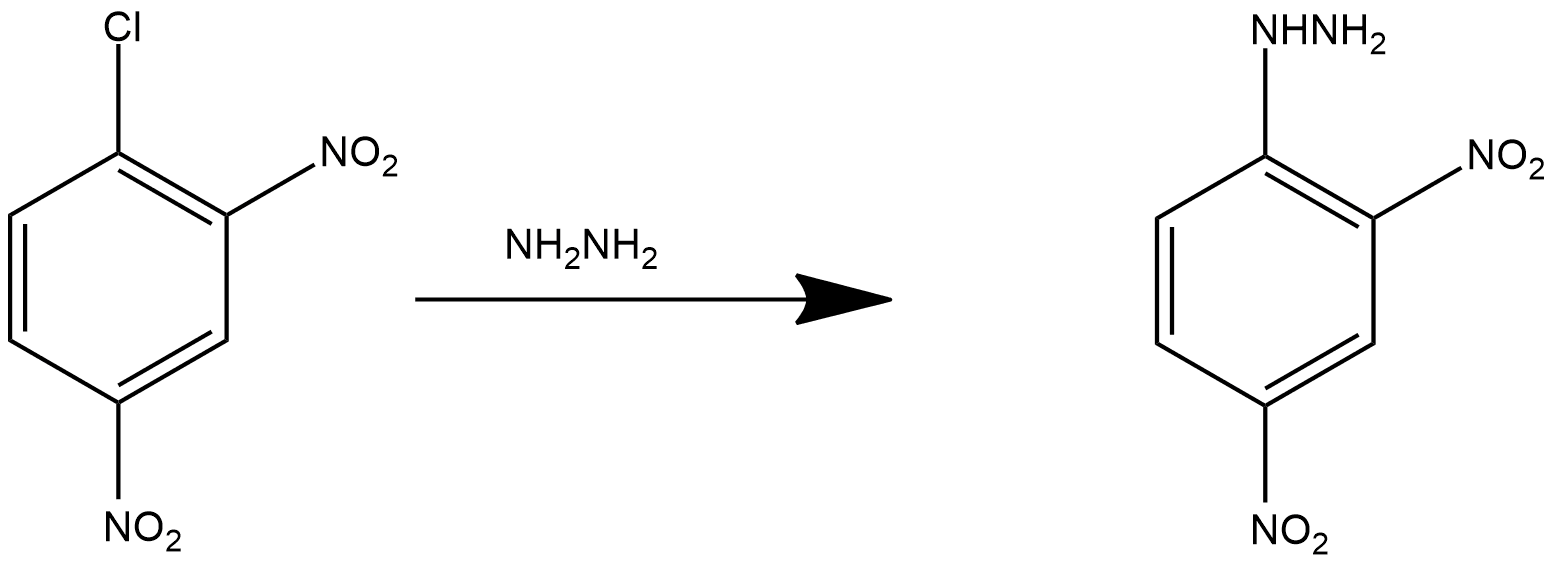
In this reaction, we can see that 2,4-dinitrochlorobenzene reacts with hydrazine and one chloro group is replaced with hydrazine group.
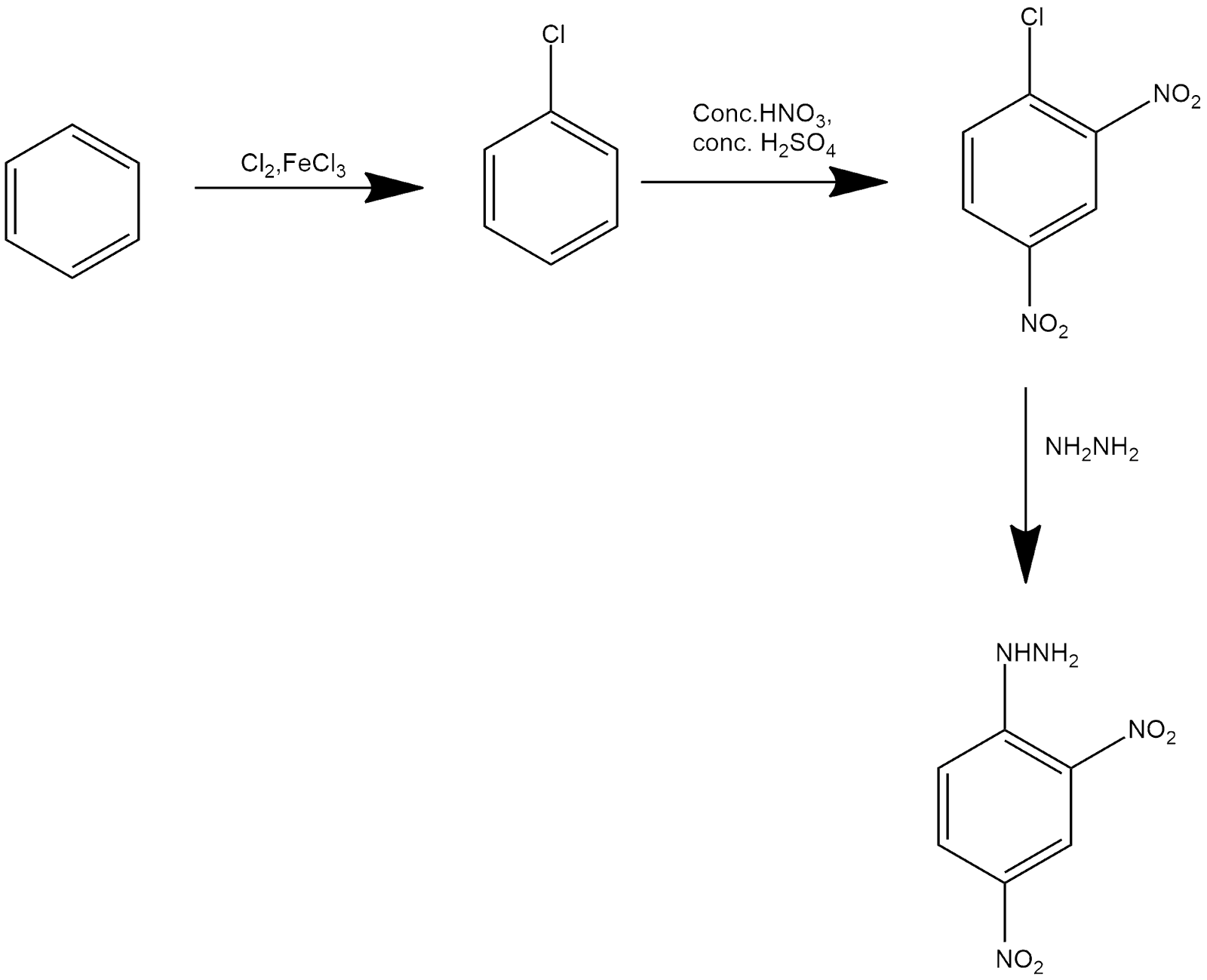
Mechanism Of Synthesis Of 2,4-DNP
The mechanism can be explained by the following three steps:
1. Chlorobenzene is prepared from benzene by chlorination, where the chlorine group is said to be an ortho and para directing group and deactivating in nature.
2. After that, we nitrate chlorobenzene with sulphuric and nitric acid. As chlorine is para or ortho directing, then the first para position is already blocked, then two nitro groups will attack on ortho and para positions as nitro is also said to be an ortho-para directing group.
3. After this, 2,4-dinitrochlorobenzene reacts with hydrazine, which replaces the chlorine group with hydrazine and gives us the desired product.
The mechanism can be shown as follows:
Brady’s Reagent
Aqueous solution of 2,4-dinitrophenyl hydrazine or 2,4-DNP is known as Brady’s reagent. This reagent reacts with carbonyl compounds, where carbonyl compounds are those that contain a carbonyl group, like aldehyde and ketone, and the carbonyl group is represented as $C=O$. While in an aldehyde carbonyl group is present as CHO, while in a ketone, $C=O$ is there and gives a colored precipitate with them. These precipitates generally show sharp melting points, and these sharp melting points will confirm the presence of carbonyl compounds in them.
2,4-DNP test is given by only those compounds which are aldehydes and ketones in nature, as when aldehyde and ketones reacts with 2,4-DNP, then it forms yellow, orange or reddish orange precipitates with 2,4-DNP, but when alcohols, which do not have carbonyl compounds gets react with 2,4-DNP, the reaction will not succeed, or we can say no reaction takes place. This test is also used to check the presence of alcohol in the body. Benzaldehyde and acetone can be distinguished by this test.
How Can We Identify Carbonyl Compounds With The Help Of 2,4-DNP?
Carbonyl compound in methanol gets easily, or we can say simply, mixed with an acid solution of Brady’s reagent. The derivatives or products formed by this reaction are of orange color and crystalline solids, which are further known by the name 2,4-dinitrophenylhydrazones. Crystals of 2,4-dinitrophenylhydrazone are further filtered and purified by the process called recrystallisation.
After drying, the melting point of the crystal has to be noted and compared with the melting point of 2,4-dinitrophenyl hydrazine. If it got matched, or we can say it gives colored precipitates, then the carbonyl group gets confirmed.
|
Related Topics Link, |
The reaction can be shown as:
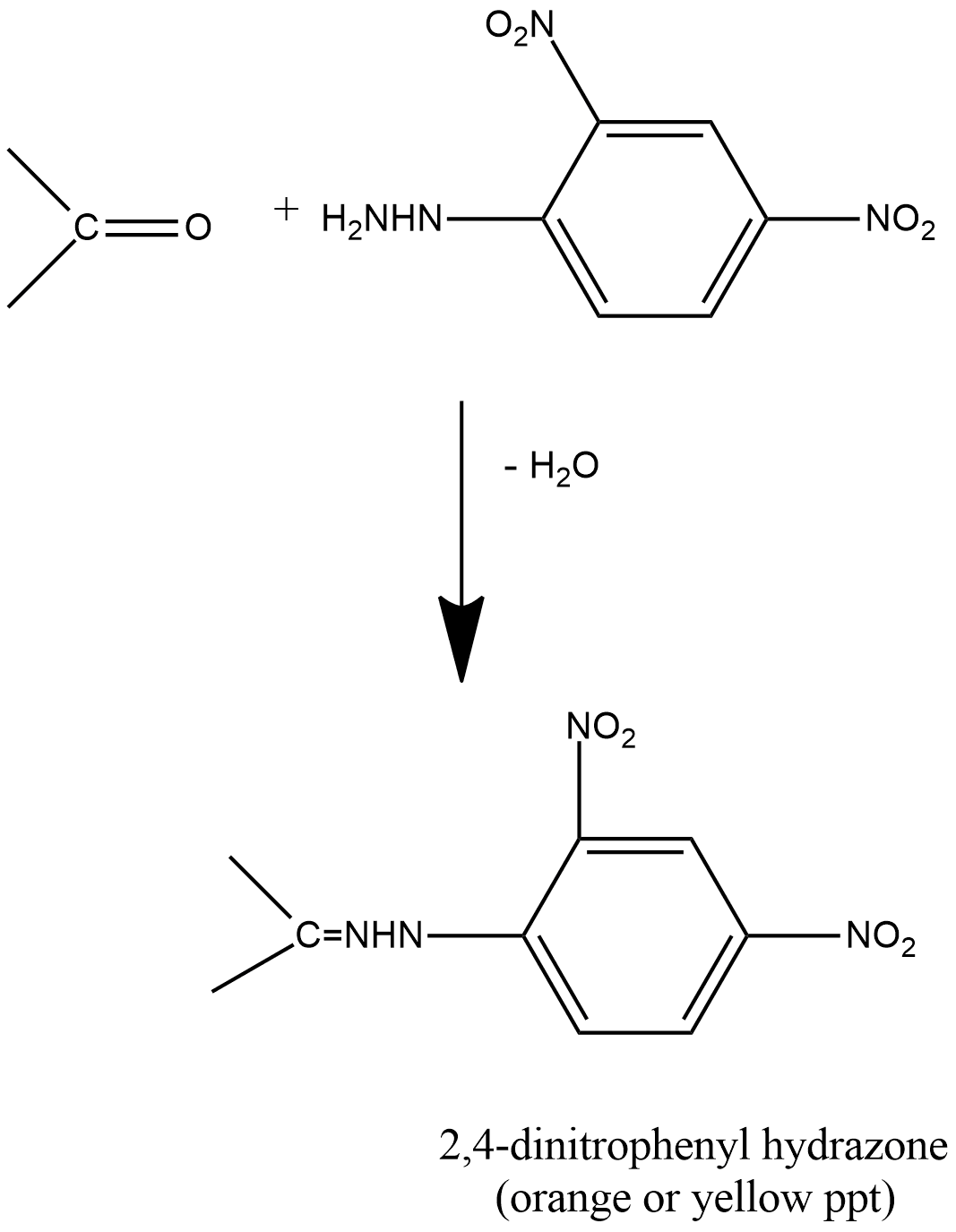
The main advantage of Brady’s test is that it is used to test whether the carbonyl compound is present or not, and the presence of the carbonyl compound in the form of aldehyde or ketone will give orange colored precipitates.
Also read :
- NCERT solutions for Class 12 Chemistry Chapter 13 Amines
- NCERT Exemplar Class 12 Chemistry solutions Chapter 13 Amines
- NCERT notes Class 12 Chemistry Chapter 13 Amines
2,4-Diphenylhydrazine Laboratory Test
This test is generally known by its abbreviation, called the 2,4-DNP test. The full form 2,4-DNP test is a 2,4-diphenylhydrazine laboratory test. Let us consider the test by first showing its image
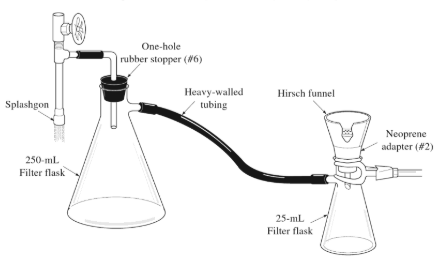
This test can be explained by the following points
1. Put 5 ml of 2,4-dinitrophenylhydrazine in a test tube.
2. Now this add 10 drops of any unknown compound and shake the test tube vigorously to mix them well, and it will form crystals. If crystals were not formed, then heat the test tube gently in a water bath at about $60^{\circ}$C for just 5-10 minutes.
3. After heating, cool the solution in an ice bath until you find the crystals. When crystals appear, wait some time to settle them down and then filter them through a Hirsch funnel, like the funnel shown in the image.
4. Now allow the crystals to dry with the help of a Hirsch funnel by drawing air through the crystals. Now, after drying the crystals, note down the melting point.
5. Take care that the crystals are dried properly and pure to note the accurate melting point. If you find the range of melting points is large, then recrystallize the crystal with ethanol.
Reaction of 2,4-DNP With Ethanal
2,4-DNP is able to react with aldehydes as well as ketones, which further produces 2,4-dinitrophenylhydrazone as a product. The reaction between 2,4-DNP and ethanal, which is an aldehyde, can be known as an elimination reaction, as in this reaction, water molecules are eliminated, and these reactions generally give orange and yellow colored solid compounds or precipitates of yellow/orange color. The melting point of these types of compounds is quite sharp, and the solution of 2,4-diphenylhydrazine is known by the name Brady’s reagent.
Also check-
Some Solved Examples
Question 1: Which of the following compounds will not react with 2,4-Dinitrophenylhydrazine (2,4-DNP) to form a yellow or orange precipitate?
A) Acetone $\left(\mathrm{CH}_3 \mathrm{COCH}_3\right)$
B) Acetaldehyde $\left(\mathrm{CH}_3 \mathrm{CHO}\right)$
C) Benzaldehyde ( $\mathrm{C}_6 \mathrm{H}_5 \mathrm{CHO}$ )
D) Ethanol $\left(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}\right)$
Solution: The 2,4-DNP test is used to detect carbonyl compounds (aldehydes and ketones). Ethanol $\left(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}\right)$ does not have a carbonyl group and hence will not react with 2,4-DNP. On the other hand, acetone, acetaldehyde, and benzaldehyde all contain carbonyl groups, and they will react with 2,4-DNP to form a yellow or orange precipitate.
Hence, the correct answer is option (4)
Question 2: Which of the following would give a red precipitate when reacted with 2,4-Dinitrophenylhydrazine (2,4-DNP)?
A) Propanal $\left(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}\right)$
B) Acetone $\left(\mathrm{CH}_3 \mathrm{COCH}_3\right)$
C) Benzaldehyde ( $\mathrm{C}_6 \mathrm{H}_5 \mathrm{CHO}$ )
D) Cyclohexanone $\left(\mathrm{C}_6 \mathrm{H}_{10} \mathrm{O}\right)$
Solution:
2,4-DNP reacts with aldehydes and ketones to form hydrazones. The color of the precipitate formed depends on the structure of the compound. Benzaldehyde $\left(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CHO}\right)$ will give a red precipitate because of the aromatic nature of the benzene ring, which stabilizes the hydrazone product, causing it to form a red color. Other compounds like propanal, acetone, and cyclohexanone typically form yellow or orange precipitates.
Hence, the correct answer is option (3)
Question 3: The reaction of 2,4-Dinitrophenylhydrazine (2,4-DNP) with an unknown compound results in the formation of a yellow precipitate. Which of the following functional groups is most likely present in the compound?
A) Alcohol
B) Aldehyde
C) Ketone
D) Carboxylic acid
Solution:
The 2,4-DNP test is specific for the carbonyl group (both aldehydes and ketones). The formation of a yellow precipitate indicates the presence of a carbonyl compound. Therefore, the unknown compound is most likely an aldehyde or ketone.
Hence, the correct answer is option B) Aldehyde or C) Ketone (Both aldehydes and ketones react with 2,4-DNP)
Question 4: Which of the following will give a positive 2,4-Dinitrophenylhydrazine (2,4-DNP) test?
A) Butan-1-ol $\left(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}\right)$
B) Propan-2-one $\left(\mathrm{CH}_3 \mathrm{COCH}_3\right)$
C) Phenol $\left(\mathrm{C}_6 \mathrm{H}_6 \mathrm{OH}\right)$
D) Ethene $\left(\mathrm{CH}_2=\mathrm{CH}_2\right)$
Solution:
The 2,4-DNP test detects carbonyl groups in aldehydes and ketones. Propan-2-one $\left(\mathrm{CH}_3 \mathrm{COCH}_3\right)$ is a ketone and will give a positive test, forming a yellow precipitate. Butan-1-ol (an alcohol), phenol, and ethene do not have carbonyl groups and will not give a positive result.
Hence, the correct answer is option (B) Propan-2-one.
Frequently Asked Questions (FAQs)
2,4-DNP stands for 2,4-dipheylhydrazine which is used to identify whether any aldehydic or ketonic group is present in any compound or not. The positive result for this test is the formation of precipitates of dinitrophenylhydrazone which is of yellow, orange or red in color.
2,4-Dinitrophenylhydrazones are said to be better derivatives as compared to phenyl hydrazones this can be explained by many different reasons. At first we can say that 2,4-dinitrophenylhydrazones and its derivatives contain higher molecular masses as compared to phenylhydrazones which increase the volume of substance and we know that those substances which are heavier in masses contain a greater tendency of becoming a solid.
Aqueous solution of 2,4-dinitrophenylhydrazone is generally known as Brady’s reagent which when react with those compounds which have carbonyl group inside them are able to give colored precipitates and in presence of alcohol it will not get reacted. So this reagent is basically used to test the presence of alcohol in the body.
2,4-dinitrophenylhydrazone.
Grignard reagent.
