Hinsberg Reagent and Test - Meaning, Preparation, Procedure, FAQs
Hinsberg Reagent and Test:
Hinsberg reagent can be described as an alternate name for benzene sulfonyl chloride. This name is given for the usage of its Hinsberg test to detect and distinguish primary, secondary, tertiary amines of a given sample. This reagent is an organosulfur compound, and the Hinsberg reagent formula (chemical formula) C6H5SO2Cl.The Hinsberg reagent is a colourless oil that has a thick consistency and is soluble in organic solvents.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Hinsberg Reagent and Test:
- What is Hinsberg Reagent?
- Preparation of Hinsberg Reagent
- The Hinsberg Test:
- Principle of Hinsberg Test:
- Mechanism of Hinsberg Test:
- Procedure for Hinsberg Test:
- Hinsberg Reaction Pathways
- Amine Determination Using Hindsberg Reagent:
What is Hinsberg Reagent?
Benzene sulfonyl chloride is also known as Hinsberg reagent formula. This name comes from its application in the Hinsberg test, which is used to detect and distinguish primary, secondary, and tertiary amines in a sample.
An organosulfur compound is what this reagent is. C6H5SO2Cl l is the chemical formula for this substance. Hinsberg reagent has the appearance of a colourless, thick oil.
This Reagent reacts with compounds that have reactive O-H and N-H bonds. It is used to make sulfonamides and sulfonamide esters (by reacting with amines) (via reaction with alcohol).
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Preparation of Hinsberg Reagent
The necessary reagent is obtained by chlorinating benzene sulfonic acid or benzene sulfonic acid salts with phosphorus oxychloride (POCl3).
Reacting benzene with chloro sulfuric acid is another technique to make the needed Heisenberg reagent (chemical reagent formula HSO3Cl). Both of these approaches for preparing the needed reagent are shown in the diagram below.
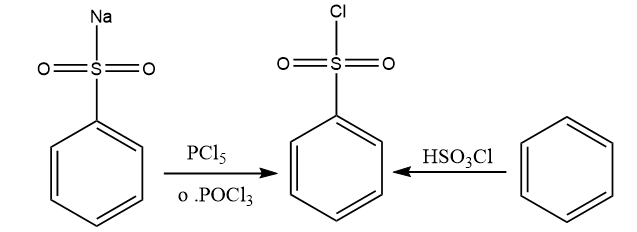
Source: Self made using Chemdraw software
The Hinsberg Test:
The Hinsberg test is a chemical reagent reaction that determines if an amine is primary, secondary, or tertiary. Oscar Heinrich Daniel Hinsberg, a German chemist, was the first to report this reaction in 1890.
The amines operate as nucleophiles in the Hinsberg test, attacking the electrophile (sulfonyl chloride). This causes the chloride to be displaced, resulting in the formation of sulfonamides. When primary and secondary amines combine to generate sulfonamides, the resulting sulfonamide is insoluble and precipitates as a solid from the solution.
Principle of Hinsberg Test:
The Hinsberg test is based on the production of sulfonamide. An amine reacts with benzene sulfonyl chloride in the Hinsberg test. Because tertiary amines can not produce stable sulfonamides, if a product occurs, the amine is either a primary or secondary amine. It's a primary amine if the sulfonamide that develops dissolves in aqueous sodium hydroxide solution.
It's a secondary amine if the sulfonamide is insoluble in aqueous sodium hydroxide and hydrogen chloride. Because it has an acidic hydrogen atom on the nitrogen, the sulfonamide of a primary amine is soluble in an aqueous base. The base sodium hydroxide absorbs this hydrogen atom, forming a sulfonamide sodium salt. The benzene sulfonyl chloride does not react with tertiary amine.
It produces an insoluble solid or oil (unreacted amine) that dissolves when acidified with hydrogen chloride, yielding a clear solution of the amine salt.
Read more :
- NCERT notes Class 12 Chemistry Chapter 13 Amines
- NCERT solutions for Class 12 Chemistry Chapter 13 Amines
- NCERT Exemplar Class 12 Chemistry solutions Chapter 13 Amines
Mechanism of Hinsberg Test:
On the highly electrophilic sulfonyl chloride derivative, the amine first interacts with benzene sulfonyl chloride in an addition-elimination process. The sulfonamide salt of sodium is formed after the chlorine and one proton from the amine are lost in a stepwise manner in the presence of sodium hydroxide.
Procedure for Hinsberg Test:
In a big test tube, add 8-10 drops of amine.
Benzene sulfonyl chloride, 10 drops
10 mL NaOH (at a concentration of 10%)
To combine, vigorously shake the ingredients (use a cork)
Examine the solution for the existence of a single layer (primary amine) or a double layer (secondary amine).
If there is a double layer, use a separatory funnel to remove the bottom aqueous layer and examine if the organic layer is soluble in 5% HCl (If it is not soluble, then secondary amine is present)
Related Topics Link |
Hinsberg Reaction Pathways
When benzene sulfonyl chloride reacts with primary amines, a sulfonamide product is formed that is alkali soluble. The following diagram depicts this reaction.
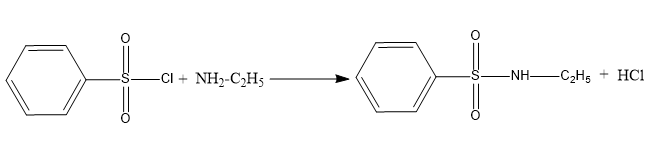
When benzene sulfonyl chloride reacts with secondary amines, a sulfonamide product is formed that is not alkali soluble. Below is an example of this type of reaction.
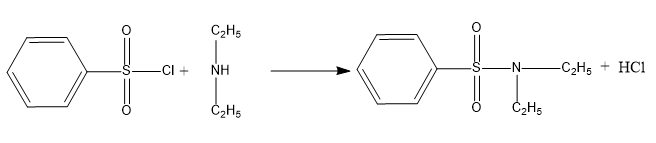
Between a tertiary amine and the benzene sulfonyl chloride reagent, no such reaction occurs. Sulfonyl chloride hydrolysis is aided by tertiary amines. This reaction produces salts that are water soluble.
As a result, the Hinsberg reagent reacts differentially with primary, secondary, and tertiary amines. These variations can be seen in the sulfonamide product's alkali solubility.
NCERT Chemistry Notes:
Amine Determination Using Hindsberg Reagent:
Hinsberg's reagent is benzene sulphonyl chloride, which may be used to discriminate between 1°, 2°, and 3° amines.
The primary amines, on the other hand, contain two active hydrogen ions, making it easier for them to react with benzene sulphonyl chloride and form salt, which may be dissolved in NaOH due to the remaining active hydrogen.
Because secondary amines only have one hydrogen ion, they react with Benzene sulphonyl chloride but not with NaOH. Because tertiary amines don't contain active hydrogen, they don't react with Benzene sulphonyl chloride.
The Hinsberg reaction is a test for primary, secondary, and tertiary amine detection. The amine is thoroughly shaken with Hinsberg reagent is in the presence of aqueous alkali in this test (either KOH or NaOH). A substrate is treated with a reagent containing an aqueous sodium hydroxide solution and benzene sulfonyl chloride. A soluble sulfonamide salt is formed by a primary amine.
The main amine's sulfonamide is precipitated after acidification of this salt. In the same process, a secondary amine forms an insoluble sulfonamide. A tertiary amine will stay insoluble if it does not react with the initial reagent (benzene sulfonyl chloride). This insoluble amine is transformed
Assume that a, b, and c represent primary, secondary, and tertiary amines, respectively.
In the aqueous phase, if R is considered as a methyl group (the total Rs, excluding hydrogen), then the order of basicity is b > a > c.
If R is any group other than a methyl group, then the basicity order is b > c > a.
When it comes to the gas phase, the basicity order can be written as c > b > a.
Also check-
Frequently Asked Questions (FAQs)
Hinsberg reagent can be described as an alternate name for benzene sulfonyl chloride. This reagent is an organosulfur compound, and the Hinsberg reagent formula (chemical formula) C6H5SO2Cl. The Hinsberg reagent is a colourless oil that has a thick consistency and is soluble in organic solvents.
Hinsberg test to detect and distinguish primary, secondary, tertiary amines of a given sample.
On the highly electrophilic sulfonyl chloride derivative, the amine first interacts with benzene sulfonyl chloride in an addition-elimination process. The sulfonamide salt of sodium is formed after the chlorine and one proton from the amine are lost in a stepwise manner in the presence of sodium hydroxide
In the test, the amines operate as nucleophiles in the Hinsberg test, attacking the electrophile (sulfonyl chloride). This causes the chloride to be displaced, resulting in the formation of sulfonamides. When primary and secondary amines can be distinguished bys combine to generate sulfonamides, the resulting sulfonamide is insoluble and precipitates as a solid from the solution.
The necessary reagent is obtained by chlorinating benzene sulfonic acid or benzene sulfonic acid salts with phosphorus oxychloride (POCl3).
Reacting benzene with chloro sulfuric acid is another technique to make the needed Hinsberg reagent (chemical formula HSO3Cl). Both of these approaches for preparing the needed reagent are shown in the diagram below.
Also Read
27 Sep'24 05:44 PM
27 Jan'24 11:41 PM
27 Jan'24 11:57 AM
26 Jan'24 10:37 PM
24 Nov'22 06:21 PM
18 Jul'22 03:38 PM
18 Jul'22 03:35 PM
