Acetamide: Chemical Formula, Structure, Uses, Properties
IUPAC name of Acetamide is ethanamide. It is an organic compound. It’s chemical formula is CH3CONH2. It is also known as Acetic acid amide. It is the simplest form of amide and it is derived from acetic acid. It is used as a plasticizer. It has applications in industries also. It is used as an industrial solvent there.DMA is a related compound and is more widely used. It’s preparation is different from acetamide. Acetamide acts as an intermediate between acetone and urea. Acetone consists of two methyl (CH3) groups that are present on both sides of the carbonyl. Urea consists of two amides (NH2) groups. Acetamide is a mineral that occurs naturally.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Other Names Of Acetamide- CH3CONH2
- Properties Of Acetamide(CH3CONH2)
- Acetamide(CH3CONH2) Structure
- Methods Of Production
- Safety Hazard Of Acetamide(CH3CONH2)
- Uses Of CH3CONH2
- Side Effects Of Acetamide(CH3CONH2)
Other Names Of Acetamide- CH3CONH2
Acetic acid amide
Acetylamine
Properties Of Acetamide(CH3CONH2)
Chemical formula of Acetamide is C2H5NO.
Molar mass of Acetamide is 59.068 g·mol−1.
It is a colourless gas or solid.
It is odourless.
Density of Acetamide is 1.159 g cm−3.
Boiling point of Acetamide is 221.2 °C and Melting point of Acetamide is 79 to 81 °C.
It’s solubility in water is 2000 g/L.
It is also soluble in ethanol, benzene, etc.
Vapour pressure of Acetamide is 1.3 Pa.
Acidity of Acetamide is 15.1.
Magnetic susceptibility of Acetamide is −0.577 x 10−6 cm3 g−1.
It’s refractive index is 1.4274.
Viscosity of Acetamide is 2.052 cP.
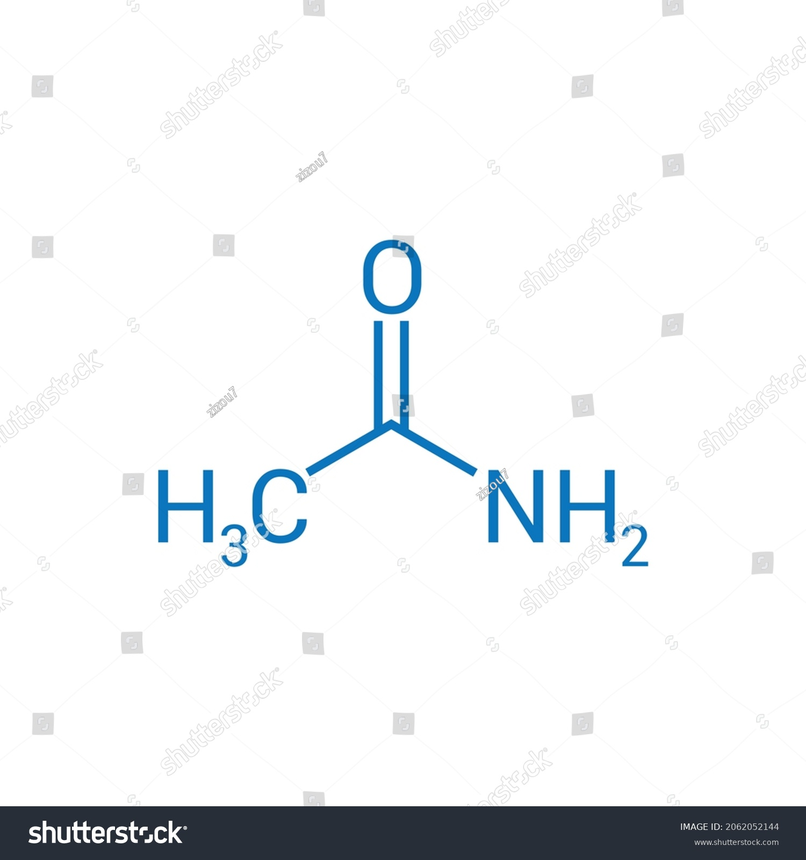
Acetamide(CH3CONH2) Structure
It is trigonal in structure.The molecule has 8 bonds where 3 bonds are non-hydrogen bonds, 1 multiple bonds, 1 double bond and 1 primary amide. The two-dimensional chemical structure of Acetamide is also known as the skeletal formula.
Methods Of Production
1.Laboratory scale
Acetamide can be formed in a huge amount by doing the ammonolysis of acetylacetone. This process is done under suitable conditions. It is generally used in reductive amination.It can be prepared from anhydrous acetic acid, dried hydrogen chloride gas and acetonitrile . It can be done with the help of an ice bath. Yield obtained is generally low , and the acetamide that is formed in this way is generated as a salt along with HCl.
2.Industrial scale
It is similar to some laboratory methods . Acetamide is obtained by dehydrating ammonium acetate. It can also be obtained by doing the hydration of acetonitrile. Byproduct of the production of acrylonitrile is Acetonitrile.
Safety Hazard Of Acetamide(CH3CONH2)
There is low toxicity in acetamide which leads to deduction in weight. When it is exposed to a high oral dose, it takes place.
It is also responsible for the mild irritation of the eyes and skin.
Fumes or toxic gases are generated by its combustion.
Corneal can also be damaged due to this.
Oral exposure can result in tumours like lymphoma and liver tumours.
Uses Of CH3CONH2
Acetamide has it’s application as a plasticizer and can be used in industries as an industrial solvent.
Acetamide is a good solvent in it’s molten form and has a wide range of applicability.
Dielectric constant of Acetamide is higher than many organic solvents. This property helps it to get dissolved in inorganic compounds.
Acetamide also has it’s applications in electrochemistry.
Acetamide is a precursor.
Side Effects Of Acetamide(CH3CONH2)
It causes headaches.
It is also responsible for Dizziness and Tiredness.
It causes the urge to urinate frequently.
It is also responsible for producing sensation and numbness.
Loss of appetite can be a side effect of acetamide.
Excitement is also caused sometimes.
Feeling of sickness can be experienced.
Diarrhoea can be caused.
Change in taste takes place.
Sense of warmth can be experienced in body parts like the face, ears, neck and trunk.
Frequently Asked Questions (FAQs)
IUPAC name of Acetamide is ethanamide. It is an organic compound. It’s chemical formula is CH3CONH2. It is also known as Acetic acid amide. It is the simplest form of amide and it is derived from acetic acid. It is used as a plasticizer. It has applications in industries also. It is used as an industrial solvent there. DMA is a related compound and is more widely used. It’s preparation is different from acetamide.
The characteristics of Acetamide(CH3CONH2) are -
Chemical formula of Acetamide is C2H5NO.
Molar mass of Acetamide is 59.068 g·mol−1.
It is a colourless gas or solid.
It is odourless .
Density of Acetamide is 1.159 g cm−3.
Boiling point of Acetamide is 221.2 °C and Melting point of Acetamide is 79 to 81 °C.
It’s solubility in water is 2000 g/L.
The uses of Acetamide(CH3CONH2) are-
Acetamide has it’s application as a plasticizer and can be used in industries as an industrial solvent.
Acetamide is a good solvent in it’s molten form and has a wide range of applicability.
Dielectric constant of Acetamide is higher than many organic solvents. This property helps it to get dissolved in inorganic compounds.
The methods of production of Acetamide(CH3CONH2) are -
1.Laboratory scale
Acetamide can be formed in a huge amount by doing the ammonolysis of acetylacetone. This process is done under suitable conditions. It is generally used in reductive amination. It can be prepared from anhydrous acetic acid, dried hydrogen chloride gas and acetonitrile.
2.Industrial scale
It is similar to some laboratory methods. Acetamide is obtained by dehydrating ammonium acetate. It can also be obtained by doing the hydration of acetonitrile. The byproduct of the production of acrylonitrile is Acetonitrile.
The side effects of Acetamide(CH3CONH2) are -
It causes headaches.
It is also responsible for Dizziness and Tiredness.
It causes the urge to urinate frequently.
It is also responsible for producing sensation and numbness.
Loss of appetite can be a side effect of acetamide.
Excitement is also caused sometimes.
Feeling of sickness can be experienced.
Also Read
27 Sep'24 05:44 PM
27 Jan'24 11:41 PM
27 Jan'24 11:57 AM
26 Jan'24 10:37 PM
24 Nov'22 06:21 PM
18 Jul'22 03:38 PM
18 Jul'22 03:35 PM