Acetate: Structure, Properties, Definition, Formula & Structure
Acetate is a chemical compound. It has the formula C2H3O2. It can also be called Acetate Ion. It is a salt that is formed by the mixture of acetic acid with some bases. The bases can be alkaline, metallic, earthy or nonmetallic. Conjugate base of acetic acid is Acetate. The abbreviation of acetate ion is AcO- or -OAc. Acetic acid is not a strong acid. It is a weak acid. It dissociates in water and releases a proton with an acetate ion. It happens at a pH of 5.5 or more. Chemical formula of Acetate is CH3COO-.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Properties Of Acetate ion
- Structure Of Acetate
- Uses Of Acetate
- Features Of Acetate
- Chemical Properties Of Acetate
.png)
When vinegar that is also known as acetic acid and baking soda that is also known as sodium bicarbonate reacts with each other, they produce an acetate ester or sodium acetate. This reaction is well known and this mixture can be used for cleaning purposes.
If we talk about biology, acetate is considered as one of the most important building blocks for biosynthesis in the human body. It includes fatty acid synthesis. It is an anion and is found in Biology.
Properties Of Acetate ion
Molecular formula of Acetate ion is C2H3O2-.
Acetic acid is the conjugate acid of Acetate ion.
Molecular weight of Acetate ion is 59.04 g/mol.
59.013 g/mol is the mass of acetate.
Complexity of Acetate ion is 25.5.
IUPAC name of Acetate ion is Ethanoate.
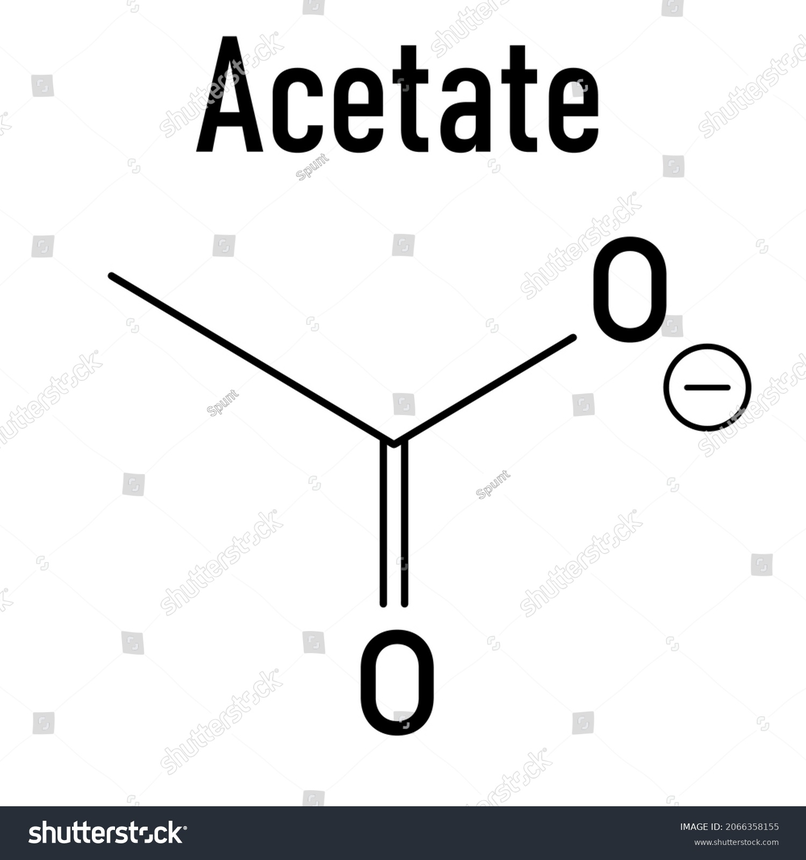
Structure Of Acetate
Acetate consists of one methyl group. This group is bonded with a carbonyl carbon. This carbonyl group is attached to another oxygen which is having a negative charge along with the methyl group. The hybridization of carbon of methyl is sp3 and it is having a tetrahedral geometry. The hybridisation of carbonyl carbon is sp2 .It is doubly bonded to oxygen and has a single bond with the oxygen that is negatively charged .Both the oxygen is equivalent . It is due to the electrons delocalization through resonance. The delocalization results in the equivalent distribution of the negative charge .
Uses Of Acetate
Acetate is used to produce vinyl acetate. It is a precursor to polyvinyl alcohol. It is also used as a component of paints.
It is also used in place of cellulose acetate. It’s production is done by using acetic acid. Production of fibres is carried out with the help of Cellulose acetate.
Many industrial solvents are acetates. It includes methyl acetate, isopropyl acetate and butyl acetate.
They are used as a fragrance product in foods.
It is also used as a solvent.
Cellulose acetate has it’s application as an eyeglass frame.
It is also found in diapers.
Potassium acetate can be used in the preservation of food.
It has it’s application in laboratories.
Aluminium acetate can be utilised as an anti stringent.
Features Of Acetate
‘Acetate’ is a compound that contains the acetate anion and a cation.
C2H3O2- or CH3COO- is the chemical formula of acetate anion.
Acetic acid is the simplest form of acetate. Hydrogen cation when combined to the acetate anion(acetic acid: CH3COOH).
Acetate is also used in biological systems in the form of acetyl.
It has it’s applications in various metabolism with the purpose to yield energy. In biology, it is used as a building block.
Chemical Properties Of Acetate
1. Acetate reacts with sodium hydroxide
When a reaction takes place between Sodium acetate and soda lime, methane and sodium carbonate are formed as a product. This type of reaction is called decarboxylation. It is one of the most common methods that is helpful in the preparation of alkanes.
2. Acetate reacts with Water
Water continuously ionises to form hydroxide anions and hydrogen cations. Sodium acetate gets dissociated in water to form sodium and acetate ions. Sodium ions do a small reaction with the hydroxide ions. And the acetate ions react with the hydrogen ions and produce acetic acid.
Frequently Asked Questions (FAQs)
Acetate is a chemical compound. It has the formula C2H3O2. It can also be called Acetate Ion. It is a salt that is formed by a mixture of acetic acid with some bases. The bases can be alkaline, metallic, earthy or nonmetallic. The conjugate base of acetic acid is Acetate.
The properties of Acetate ions are -
Molecular formula of Acetate ion is C2H3O2- .
Acetic acid is the conjugate acid of Acetate ion.
Molecular weight of the Acetate ion is 59.04 g/mol.
59.013 g/mol is the mass of acetate.
The uses of Acetate are-
Acetate is used to produce vinyl acetate. It is a precursor to polyvinyl alcohol. It is also used as a component of paints.
It is also used in place of cellulose acetate. It’s production is done by using acetic acid. Production of fibres is carried out with the help of Cellulose acetate.
Many industrial solvents are acetates. It includes methyl acetate, isopropyl acetate and butyl acetate. It is also used in fragrance.
The chemical properties of Acetate are -
1. Acetate reacts with sodium hydroxide
When a reaction takes place between Sodium acetate and soda lime, methane and sodium carbonate are formed as a product. This type of reaction is called decarboxylation .
2. Acetate reacts with Water
Water continuously ionises to form hydroxide anions and hydrogen cations. Sodium acetate gets dissociated in water to form sodium and acetate ions. Sodium ions do a small reaction with the hydroxide ions. And the acetate ions react with the hydrogen ions and produce acetic acid.
The features of Acetate are -
Acetate is a compound that contains the acetate anion and a cation.
C2H3O2- or CH3COO- is the chemical formula of acetate anion.
Acetic acid is the simplest form of acetate. Hydrogen cation when combined to the acetate anion(acetic acid: CH3COOH).
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM

