Oxalic Acid - Formula, Uses, Structure, Properties, FAQs
What is Oxalic Acid?
Oxalic acid is a colourless, crystalline and poisonous organic compound which is a member of the family of carboxylic acids and is the simplest dicarboxylic acid. The IUPAC name of oxalic acid is ethanedioic acid and it is represented by a chemical formula H2C2O4. The equivalent weight of oxalic acid or equivalent mass of oxalic acid (molecular weight of oxalic acid) or the molar mass of oxalic acid is is 90 g/mol and it is generally present in its crystalline hydrated form i.e., as oxalic acid dihydrate with chemical formula;
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Oxalic Acid?
- Oxalic Acid Structure
- Preparation of Oxalic Acid
- Calculation of equivalent weight of crystalline oxalic acid
- Physical Properties of Oxalic Acid
- Chemical Properties of Oxalic Acid
- Uses of oxalic acid
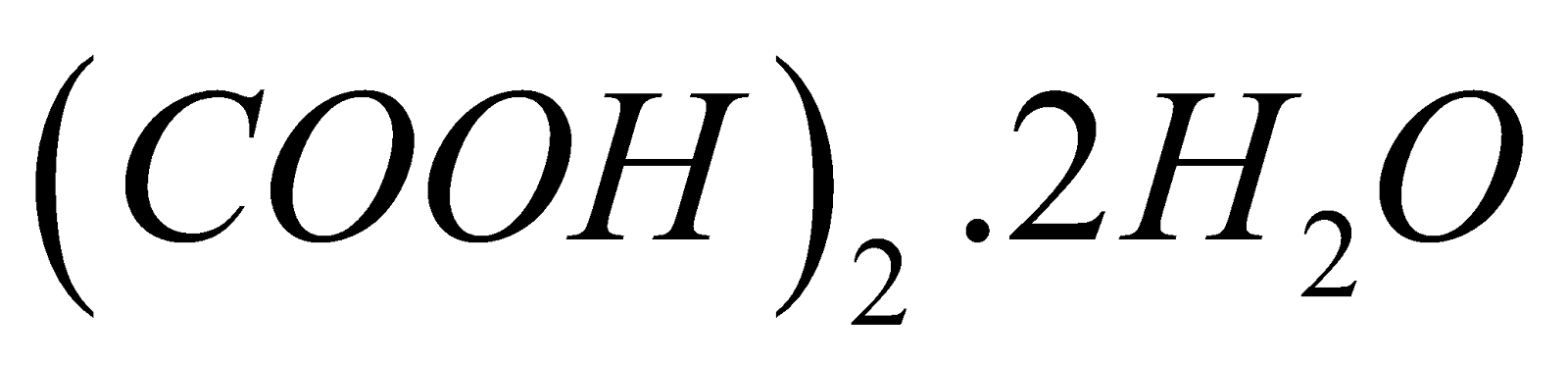
Oxalic Acid Structure
The molecular formula of oxalic acid is H2C2O4 and structurally it can be represented as follows:
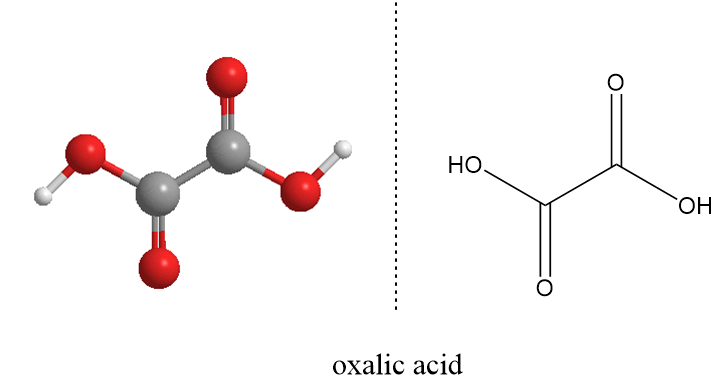
The oxalic acid exists in two crystalline structures, in one the hydrogen bonding which leads to the chain like structure of the molecule and in other, the hydrogen bonding pattern form a definite sheet like structure.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Preparation of Oxalic Acid
Oxalic acid occurs as potassium hydrogen oxalate in the wood sorrel, tomatoes and rhubarb. The insoluble calcium oxalate is found in some stone deposits in gall bladder and kidney in the human body. The oxalic acid can be synthesised as per following methods:
1. Industrial manufacture of oxalic acid:
Firstly, sodium oxalate is formed by heating sodium formate to 400°C as per following reaction:
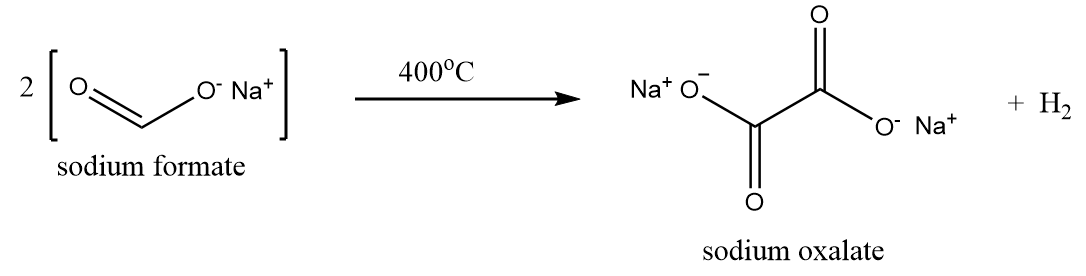
Then the sodium oxalate formed, is dissolved in the solution of water and calcium hydroxide. Solution of sodium oxide is formed along with the removal of calcium oxalate as a precipitate as per following reaction:
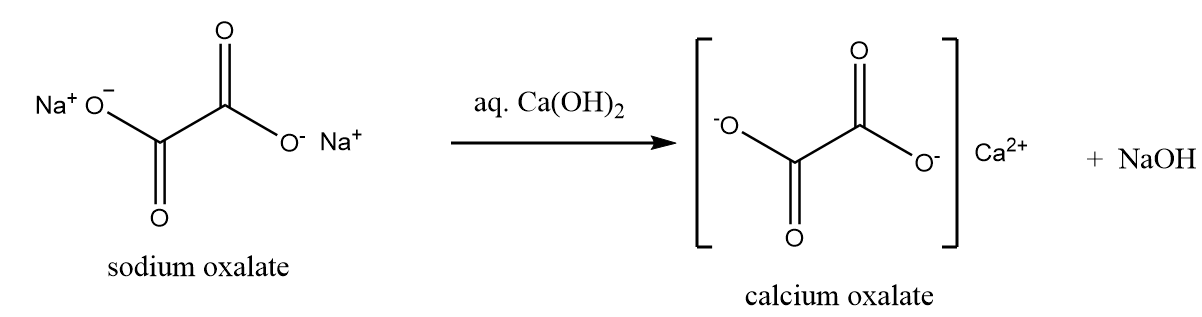
The solution is then filtered and treated with the calculated amount of dilute sulphuric acid to liberate oxalic acid. The reaction involved in the process is represented as follows:
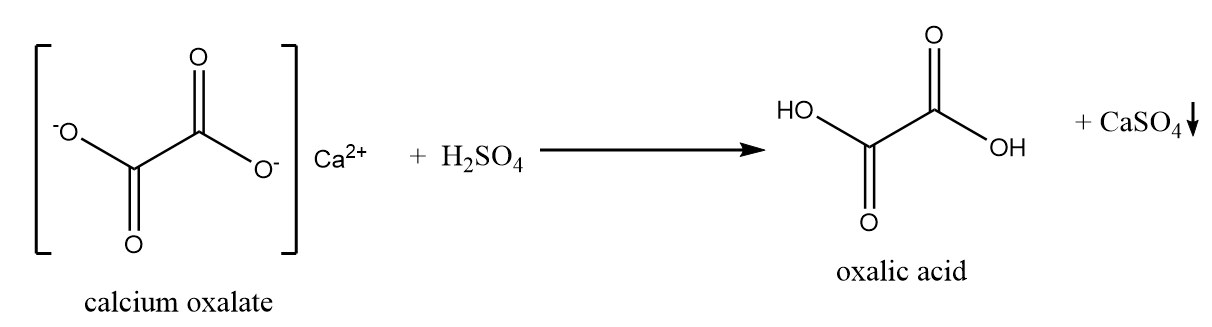
Calcium sulphate formed in the reaction is filtered out as a precipitate and oxalic acid is crystallised from the filtrate as dihydrate.
2. Laboratory method for preparation of oxalic acid:
In the laboratories, oxalic acid is prepared by oxidation of sucrose with concentrated nitric acid in the presence of a catalyst i.e., vanadium pentoxide. Basically, the -CHOH.CHOH units present in sucrose molecules split out and get oxidized to form oxalic acid. The reaction involved in the process is represented as follows:
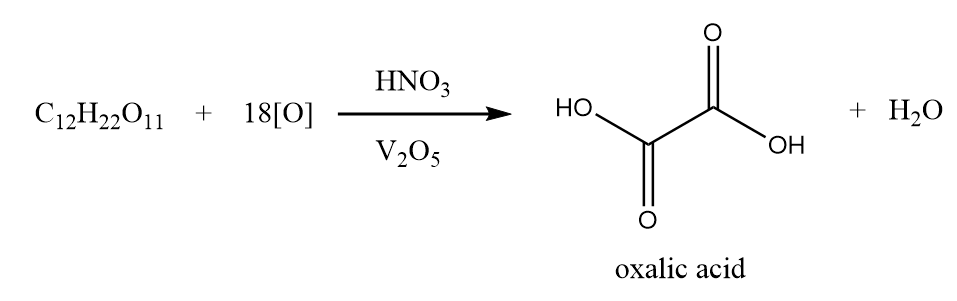
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 2 Solutions
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 2 Solutions
- NCERT notes Class 12 Chemistry Chapter 2 Solutions
Calculation of equivalent weight of crystalline oxalic acid
Oxalic acid is a dibasic acid which means it has a tendency to donate two hydrogen ions (H+), so the n-factor or valency factor of oxalic acid is 2. Therefore, the equivalent mass of oxalic acid can be calculated as per following formula:
Equivalent mass=molar mass / n-factor
As we know that the oxalic acid generally exist in its hydrated form and molar mass of oxalic acid dihydrate formula mass is 126gmol-1. Substituting the values, the equivalent mass of oxalic acid will be as follows:
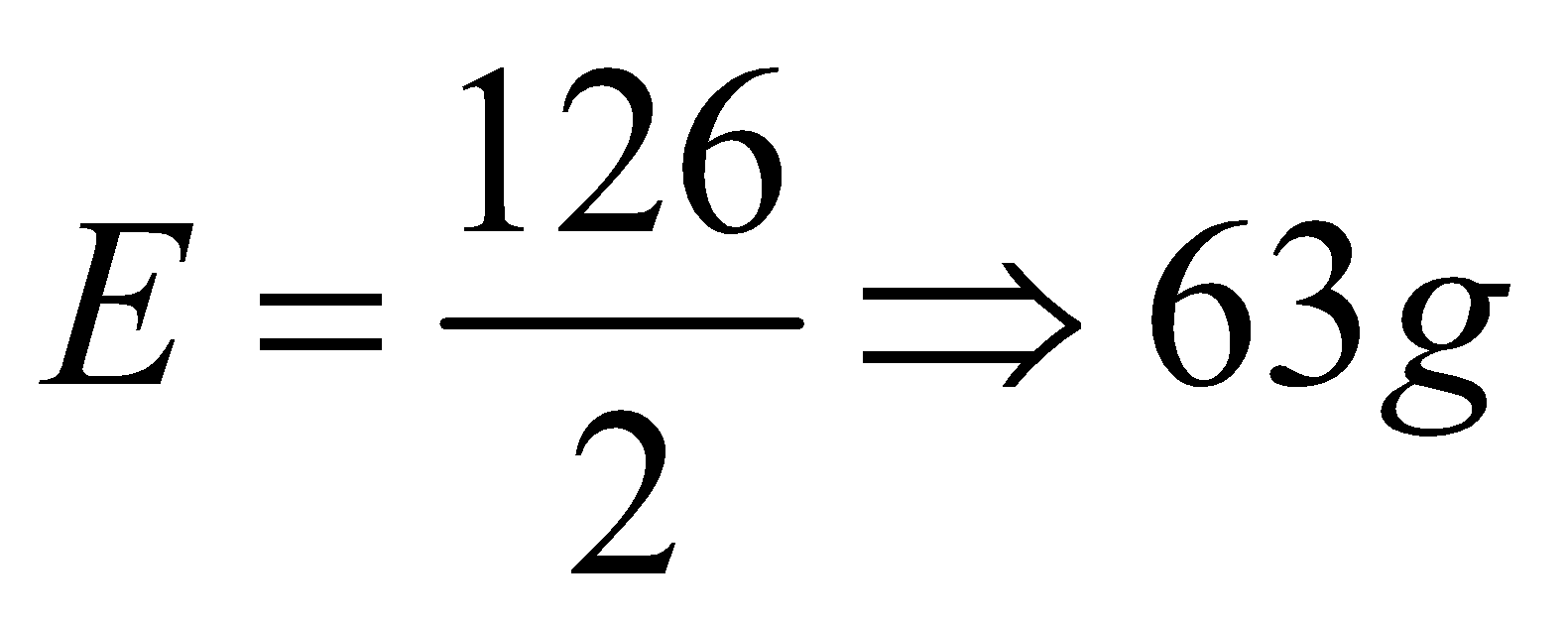
Thus, equivalent weight of oxalic acid is 63g.
Physical Properties of Oxalic Acid
At its crystalline form, it exists as colourless prismatic crystals of oxalic acid dihydrate i.e., (COOH)2.2H2O. The oxalic acid dihydrate melts at 101.5ºC while the anhydrous oxalic acid melts at 189.5ºC. The hydrate acid becomes anhydrous when it is heated to a temperature of 150ºC. Oxalic acid is an active poison, depressing the central nervous system and causes malfunction of kidneys.
Chemical Properties of Oxalic Acid
The oxalic acid is composed of two carboxyl groups bonded via single bond in direct union. It undergoes all the usual reactions of COOH group twice. Also, the oxalic acid undergoes some peculiar reaction.
Related Topics Link, |
1. Formation of mono and di-derivatives:
Oxalic acid is a much stronger acid as compared to acetic acid and have a tendency to readily form a series of salts, esters, acid halides and amides. The examples are shown as follows:
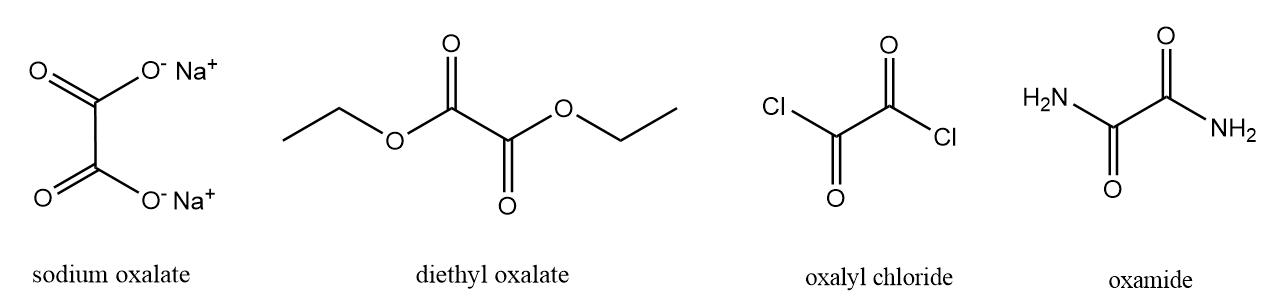
2. The action of heat on oxalic acid:
When oxalic acid is heated at 150ºC, decarboxylation takes place and formic acid is formed along with the removal of carbon dioxide. The reaction is represented as follows:
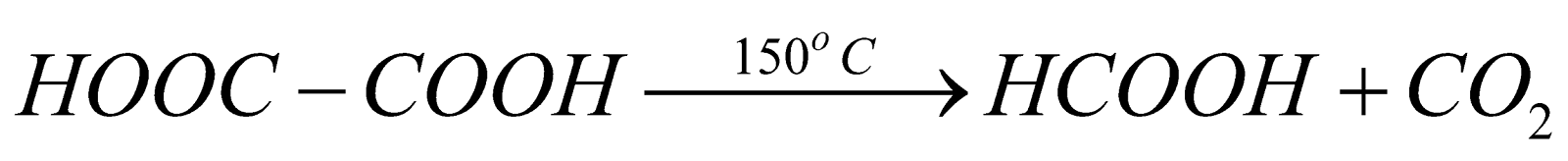
3. Reaction of oxalic acid with sulphuric acid:
When oxalic acid is heated with concentrated sulphuric acid, it’s decomposed to form carbon dioxide and carbon monoxide along with the removal of water. The reaction takes place as follows:
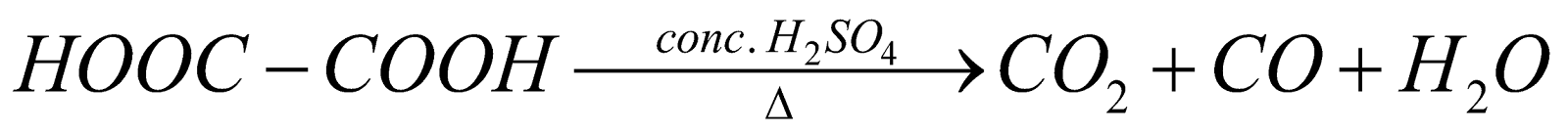
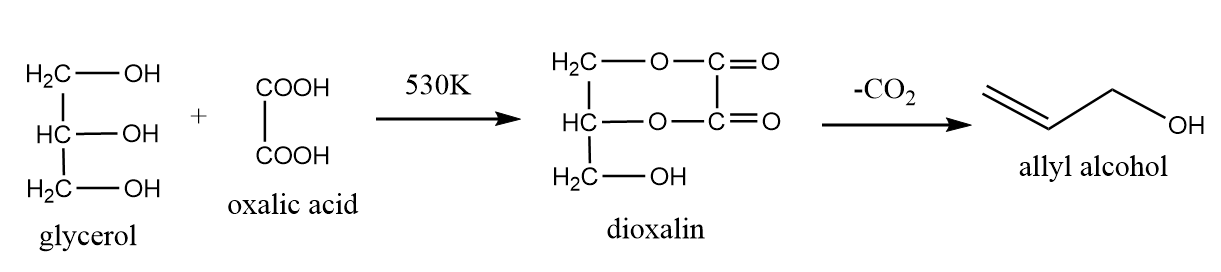
4. Reaction of oxalic acid with glycerol:
Oxalic acid react with glycerol to form formic acid depending on the experimental conditions. If oxalic acid reacts with glycerol at 530K, then allyl alcohol is formed as per following reaction:
5. Action with KMnO4:
Oxalic acid readily oxidized in the presence of acidified potassium permanganate to form carbon dioxide and water as per following reaction:
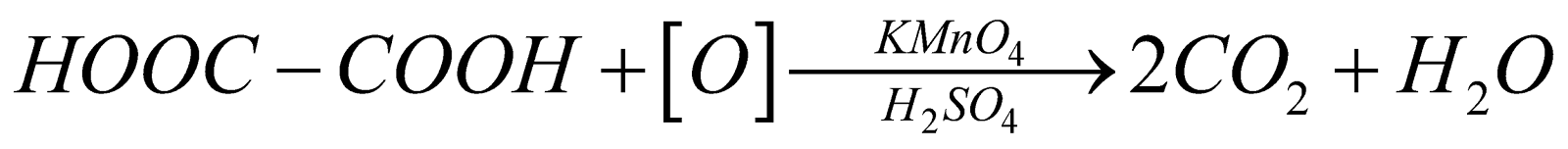
Uses of oxalic acid
Some important uses of oxalic acid are discussed below:
1. It is used for removing ink stains as well as for bleaching straw for hats, since it has a tendency to reduce brown ferric compounds to soluble and colourless ferrous salts.
2. Oxalic acid is used as a mordant in dyeing and calico printing.
3. It is used in the manufacture of inks and metal polishes.
4. It is widely used in the synthesis of allyl alcohol and formic acid in the laboratory.
5. It is also used in redox titrations.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Oxalic acid is a weak acid also known as soft acid. It is weaker than hydronium ion i.e., H3O+ but stronger than acetic acid, sulphuric acid, etc. due to the carboxyl group present in the molecule.
Oxalic acid present in spinach.
As oxalic acid is a dibasic acid i.e., it has a tendency to donate two hydrogen atoms. Thus, it is a polar molecule and capable of forming hydrogen bonds with water molecules. Since it is a polar molecule, it is soluble in all polar solvents.
Advantages-
a. Having adequate levels of oxalic acid in blood eliminates all abnormal cells effectively with no harmful side effect and thus, helps in curing cancer.
b. Oxalic acid can be reused and is very useful in beekeeping.
Disadvantages-
a. Oxalic acid can cause extensive damage to plants.
b. Oxalic acid in its crystalline form is poisonous.
Ethylene glycol
b. Sucrose
c. Ethanedioic acid
d. Propane
Ans. Correct answer is option (B).
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM
