Acetone Formula - Structure, Properties, Uses, FAQs
Acetone is a colorless, volatile liquid having a sweet and fruity odor. It is the simplest ketone which is represented by the chemical formula C₃H₆O. It is widely used as a solvent in nail polish removers, paint thinners, and cleaning agents, as well as in the production of plastics and other chemicals. Due to its effectiveness in dissolving many substances, acetone plays a crucial role in various industrial and laboratory settings. Propanone is another name for this chemical molecule.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Acetone Formula And Structure:
- Acetone/Propanone Structure:
- Propanal
- Preparation of acetone:
- Cumene process of acetone preparation:
- Properties of acetone:
- Use of acetone:
- Acetone's Industrial Applications:

Acetone Formula And Structure:
A chemical comprises two types of formula: chemical formula and structural formula. Chemical formulae show how the atoms inside a molecule are bound to one another, while structural formulas indicate how the atoms inside a molecule are bonded to one another.
Acetone:
Acetone is the common name of propanone. The acetone IUPAC name of acetone is Propan-2-one
What is the formula of acetone and Other Names Of Acetone?: Other names of acetone include dimethyl ketone, 2-propanone, and beta-ketopropane. Three carbon atoms, six hydrogen atoms, and one oxygen atom make up acetone. Because it contains a carbonyl group, it is classified as a ketone. As a result, the acetone or propanone formula or molecular formula of propanone is- C3H6O and acetone structural formula or acetone molecular formula is given as CH3-CO-CH3.
Also read :
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Acetone/Propanone Structure:
The acetone structural formula is shown as:
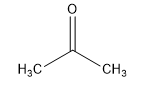
The above diagram shows propanone structural formula
The 2D chemical structure depiction of acetone is shown as:
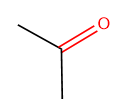
Individual bonds in the compound is represented as:
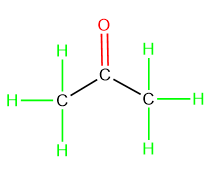
The 3-dimensional structure of acetone is shown as:
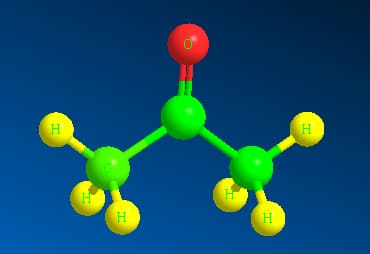
Here the green colour represents the C atoms whereas yellow represents the hydrogen atoms and red represents Oxygen atom. In the space filling structure acetone appears to be :
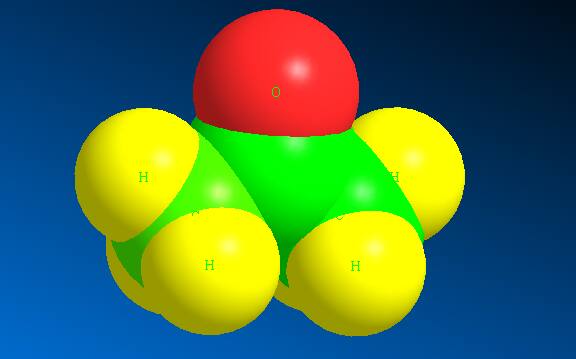
(Source: Self made using Chem3D software)
Here the green colour represents the C atoms whereas yellow represents the hydrogen atoms and red represents Oxygen atom.
Propanal
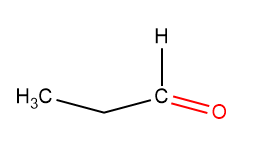
When an aldehydic group replaces the ketonic group –CO of propanone –CHO the resulting compound is called Propanal. The chemical formula of Propanal is CH₃CH₂CHO. Propanal is also called propionaldehydes and is a three-carbon aldehyde compound.
Propanal Structure:
The Propanal structure is found to be:
Formation And Existence Of Acetone:
Acetone is a byproduct of metabolism that occurs naturally in the body. Acetone is a breakdown product of saturated fat metabolism that can be found in plants, trees, wildfires, and automobile exhaust. This substance is generally present in extremely minute concentrations in urine and blood; however, diabetics' urine and blood may include greater amounts. In high doses, acetone is poisonous. Acetone must be kept in a jar with a tight lid because it is a highly flammable liquid which must be kept away from any stoves or fire sources. If you need to keep a significant amount of acetone, store it in a fireproof container.

NCERT Chemistry Notes :
Preparation of acetone:
Propylene is currently used to make acetone, either directly or indirectly. During the cumene process, over 83 percent of acetone is created. There are also other earlier methods for producing acetone. The laboratory preparation of acetone is as follows:
CH3COO2Ca→CH3COCH3+CaCO3
Heat anhydrous calcium acetate with acetone to make acetone in the lab. In a retort with a water condenser and a receiver, combine the fused calcium acetate and small iron filings. When acetone embodies over and settles in the receiver, the reaction is gradually heated. When you get colored crystals, you shake the distillation with a saturated sodium thiosulfate solution to refine the acetone you get.
These crystals provide acetone an aqueous solution after distillation with a saturated sodium carbonate solution, which would be dried over anhydrous calcium chloride and redistilled to just get pure acetone.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Cumene process of acetone preparation:
Because of its cheaper costs, the cumene route, in which acetone is coproduced with phenol, is the preferred technology; about 90% of acetone is made this way. The reaction of propylene with benzene in the presence of phosphoric acid-based catalysts or, more recently, zeolite catalysts is the major technique for production. Cumene is oxidized to cumene hydroperoxide in the liquid phase, which is then split into phenol and acetone in the presence of sulphuric acid. For every ton of phenol, 0.62 tons of acetone is generated.
Properties of acetone:
Acetone is a colorless, transparent liquid with a sweet taste odour.
The molecular weight or the molecular mass of acetone is found to be 58.08g/mol.
The boiling point of acetone is estimated to be 56 °C.
The melting point of acetone is -94.7°C.
Acetone has a density of 784 g/cm3.
0°F is the flash point of acetone.
It has a lower density than water.
Vapors have a higher density than air.
Precautions that should be taken for working with acetone: If not handled properly, acetone can be life-threatening. You can create a safe atmosphere for yourself and others by taking a few easy actions. When working with acetone, you must follow these steps:
Make sure you're shielded and wearing chemical safety eyewear.
Avoid extended exposure by using chemical-resistant clothing such as gloves, boots, and aprons.
Make that the area is safe.
Ascertain that the area is well ventilated.
Ensure the area is dried enough to prevent the liquid from soaking into the surface.
Use of acetone:
- It is a colorless liquid used during the production of plastics and other industrial products
- Acetone can also be used in a limited number of domestic products, such as cosmetics and personal care items
- It is being in the formulation of nail polishes.
To produce a variety of substances, including chloroform, sulphonal (a hypnotic), an artificial smell, cordite (a smokeless powder), and so on.
Essential oils are extracted using this.
To clean the glass apparatus, use as a solvent.
As a cleaning agent for removing tough greases from fabrics to automotive engines and other motor vehicles.
Acetone's Industrial Applications:
Acetone is used to make acetylene, cellulose acetate, cellulose nitrate, celluloid, varnishes, and lacquers, among other things.
It is used in the petroleum industry to improve the efficiency of natural gas fuel.
To remove oil from the water's surface in the event of an accident spill, protecting marine life that would otherwise perish due to oxygen loss.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Frequently Asked Questions (FAQs)
The proper name for acetone is propan-2-one. It is a simple ketone with the chemical formula C₃H₆O.
The acetone formula in chemistry is C₃H₆O.
Acetone is commonly used as a solvent for cleaning, paint thinning, and in nail polish removers.
The name of C₃H₆O is acetone or propan-2-one.
Acetone can be flammable, cause skin and eye irritation, and may lead to respiratory issues if inhaled in high concentrations along with this, prolonged exposure can also affect the nervous system.
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM

