Alcohol Phenol and Ether - Classification, Preparation with FAQs
Alcohol Phenol Ether
The alcohol, phenol and ether are classes of compounds containing a single carbon-oxygen bond that have the following general structures.
Alcohol R-O-H
Phenol 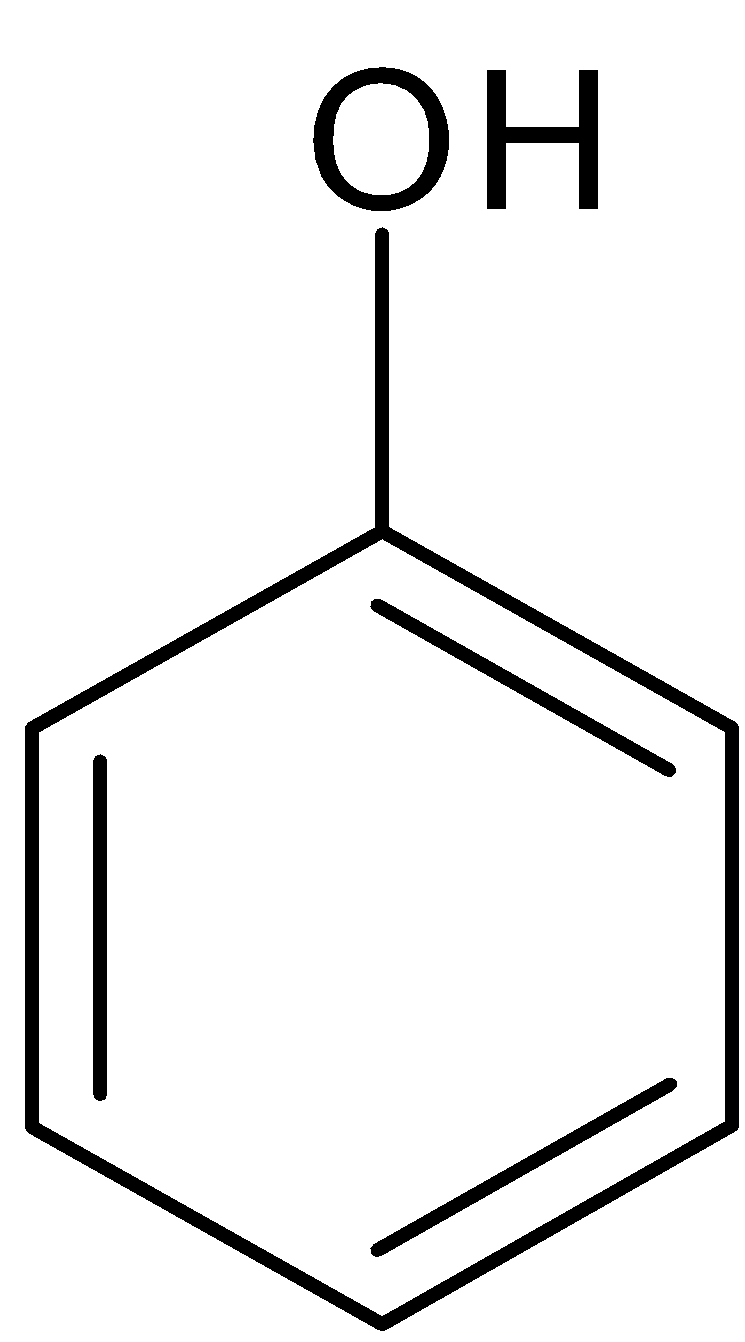
Ether R-O-R’ (R/R’ may be aromatic)
Ether, alcohol, and phenol are chemical molecules that are widely used in a variety of industries as well as in everyday life.
When a saturated carbon atom is linked to a hydroxyl (-OH) group, alcohol is produced. When a hydrogen atom in a benzene molecule is replaced by the -OH group, phenol is produced. It means a phenol contains a hydroxyl group (-OH) directly attached to carbon atom present in an aromatic compound. In alcohol one or more hydroxyl group is directly attached to carbon atom present in an aliphatic compound.
When an oxygen atom is joined to two alkyl or aryl groups, ether is produced. It means substitution of an alkoxy or aryloxy group (R–O/Ar–O) for a hydrogen atom in a hydrocarbon such as CH3OCH3 (dimethyl ether).
Also read -
Alcohols
Nomenclature of Alcohol
The word "alcohol" is added to the name of the alkyl group, for example, methyl alcohol, ethyl alcohol, and so on.
IUPAC - The suffix ‘ol' added to the name of an alkane, for example, methanol (CH3OH), ethanol (C2H5OH), and so on.
Classification of Alcohols or types of alcohols
Alcohols are classified into mono-, di-, tri- or polyhydric compound. This classification is done depending on the hydroxyl groups attached to the compound. Monohydric alcohols are those that have only one -OH group. For instance, CH3CH2-OH.Two -OH groups are found in dihydric alcohols, example is 1,2-Ethandiol.. There are three -OH groups in trihydric alcohols, example is 1,2,3-Propantriol.
Monohydric CH3-CH2-OH
Dihydric ![]()
Trihydric 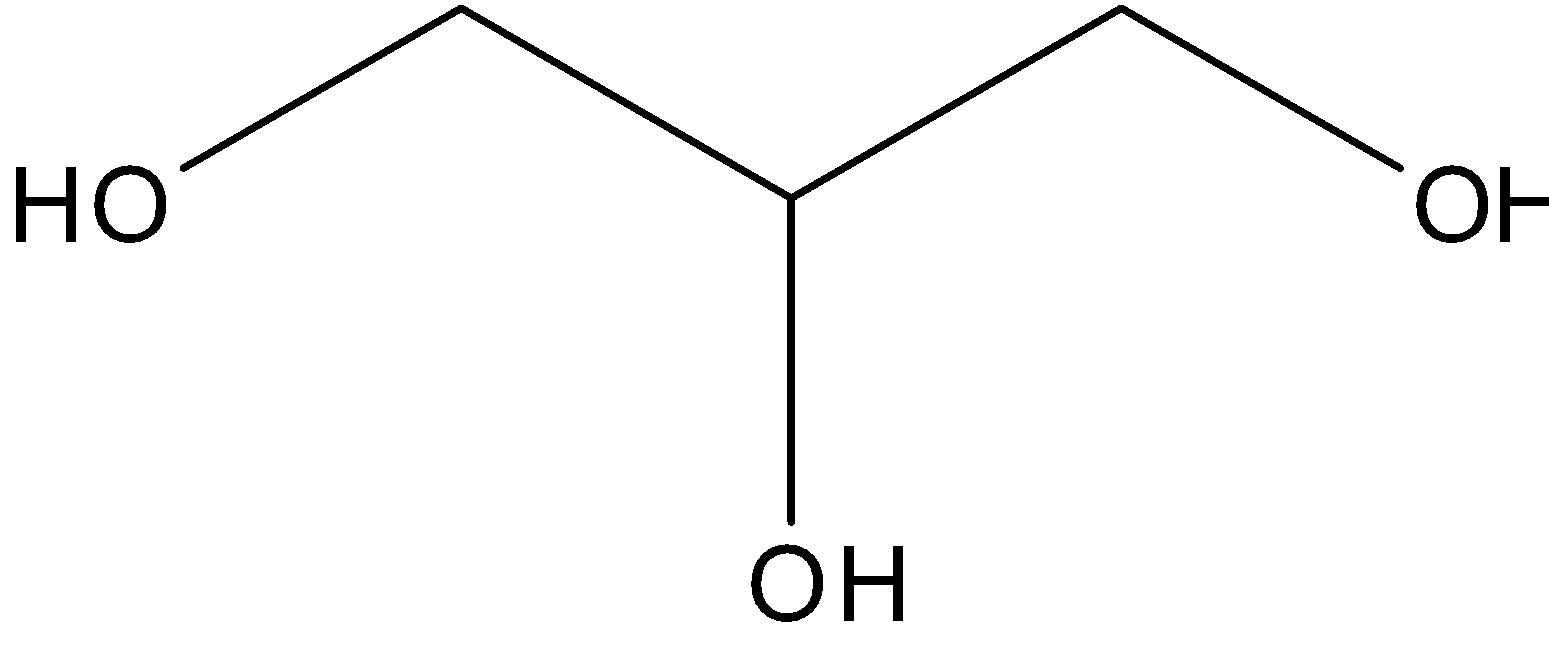
Monohydric alcohols are further classified depending on the hybridization of carbon atom to which hydroxyl group is attached.
The carbon containing sp3hybridisation- In this hydroxyl group is attached to the carbon atom having sp3 hybridisation. They are further divided into the following categories: primary, secondary, and tertiary alcohols: The –OH group is connected to the primary, secondary, and tertiary carbon atoms in these three forms of alcohols, as shown below:
R-CH2 –OH R2-CH-OH R3-C-OH
(Primary) (Secondary) (Tertiary)
Allylic alcohols: In these type alcohols, the —OH group is connected to a sp3 hybridised carbon adjacent to the carbon-carbon double bond that is to an allylic carbon.
Benzylic alcohols: In these types of alcohols, the —OH group is connected to a sp3—hybridised carbon atom next to an aromatic ring. e.g. CH2=CH-CH-OH
Vinylic alcohol: In these type alcohols, the – OH group connected to C (sp2) with C=C, e.g. CH2=CH-OH
- Compounds containing Csp2-OH bond: The —OH group is attached to a carbon-carbon double bond, such as a vinylic carbon or an aryl carbon, in these alcohols. Vinylic alcohols are another name for these alcohols.
Vinylic alcohol: CH2 = CH – OH
Also read :
- NCERT notes Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- NCERT solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 11 Alcohols, Phenols and Ethers
Preparation of Alcohol
- From Alkenes:
a. Acid-catalyzed hydration- Markonikov's rule-based H2O addition.
b. Hydroboration-oxidation- Reaction with diborane to produce trialkyl boranes, which are then oxidised with H2O2 and NaOH (aq).
The end result is the total opposite of Markonikov's rule.
- From Grignard Reagents:
Grignard reagent(R-Mg-X) is used to react with aldehydes and ketones. The Grignard reagent is added nucleophilically and then hydrolysed.
HCHO + RMgX → RCH2OMgX + H2O → RCH2OH + Mg(OH)X
Chemical Reactions of Alcohol
- Alcohol reacts as a nucleophile in the following reactions (O-H bond cleavage):
a. Metals react with alkoxides and H2 to create the corresponding alkoxides and H2.
2R-O-H + 2Na → 2R-O-Na + H2
b. Esterification: Alcohols form esters when they react with carboxylic acids, acid anhydrides, and acid chlorides.
- Alcohol interacts as an electrophile (C-O bond cleavage) in the following reactions:
a. Alkyl halides are formed by reacting with HX. The Lucas test, which distinguishes between 1°, 2°, and 3° alcohols, is based on this.
As 3° alcohols easily form halides, turbidity is formed almost immediately.
b. Alkyl halides are formed by reacting with PX3.
c. Dehydrate to create alkene in the presence of protic acid, e.g., Ethanol reacts with H2SO4 at 443 K to form ethylene.
Dehydration of 2° and 3° alcohols occurs under more mild conditions. The order of dehydration is:
3° > 2° > 1°
d. Aldehyde and ketone formation via oxidation or dehydrogenation
Aldehydes are formed from primary alcohols.
Carboxylic acid is formed directly by strong oxidising agents (KMnO4).
Aldehydes are made with CrO3 and PCC (pyrimidine chlorochromate).
When secondary alcohols are oxidised by CrO3, they produce ketones. Tertiary alcohols are unaffected by oxidation. Dissociation of the C-C bond occurs when KMnO4 is applied at a higher temperature, resulting in a variety of carboxylic acids with fewer carbon atoms.
e. When alcohols are heated with Cu at 573 K, they dehydrate.
1° alcohol → Aldehyde
2° alcohol → Ketone
3° alcohol → Alkene (Dehydration)
Phenols
Nomenclature of Phenols
Phenol (C6H5OH) is the most basic. It is the common name, and it is also accepted by the International Union of Scientific Organizations (IUPAC).The OH group's position is indicated by the letters o (ortho), m (meta), p (para), or by numbering the cyclic carbons.
For example, 2-Methylphenol is o-Cresol, while Benzene-1,2-diol is Catechol.
Classification of Phenols or types of phenol
Monohydric : Monohydric phenols are phenols with only one -OH group.
Dihydric : Two -OH groups are found in dihydric phenols. They can be ortho, meta or para derivatives.
Trihydric : Three -OH groups are found in trihydric phenols.
Preparation of Phenols
- From Haloarenes:
Chlorobenzene is converted to sodium phenoxide by reacting with NaOH, which is subsequently converted to phenol by reacting with acid.
C6H5Cl + NaOH → C6H5ONa + HCl → C6H5OH
- From Benzene sulphonic Acid:
Sulphonation of benzene with oleum is the initial stage. The resulting benzene sulphonic acid is heated with molten NaOH to generate sodium phenoxide, which is subsequently acidified to get phenol.
- From Cumene:
Cumene (isopropylbenzene) is oxidised to cumene hydroperoxide, which is then treated with dilute acid to produce phenol. The reaction produces acetone as a by-product.
Chemical Reaction of Phenols
- Nucleophilic reactions using phenol (O-H bond cleavage):
a. Phenol becomes sodium phenoxide when it interacts with metal or aqueous NaOH.
b. Esterification: Phenols create esters when they react with carboxylic acids, acid anhydrides, and acid chlorides.
Salicylic acid + Acetic anhydride → Aspirin (Acetylsalicylic acid)
- C-O bond cleavage reactions:
Benzene is formed when phenol interacts with zinc dust.
C6H5OH + Zn → C6H6 + ZnO
Phenols are categorised into three categories based on the amount of hydroxyl groups attached.
Ethers
Nomenclature of Ethers
Common Name – Ethyl methyl ether, Diethyl ether, and so on are examples of the word ‘ether’ following the names of the alkyl groups in alphabetical sequence.
IUPAC Name – A hydrocarbon derivative known as an alkoxy or aryloxy derivative. The parent hydrocarbon is the bulkier group, such as methoxymethane, methoxybenzene, and so on.
Classification of Ethers or types of ethers
Ether can be categorised into two categories based on the type of alkyl or aryl groups connected to the oxygen atom.
Symmetrical ether: The alkyl or aryl group linked to each side of the oxygen atoms is the same in symmetrical ether, also known as the simple ether. CH3OCH3, C2H5OC2H5, and so on are examples.
Unsymmetrical ether: The alkyl or aryl groups linked to either side of the oxygen atoms are not the same in unsymmetrical ether, often known as the mixed either. CH3OC2H5, C2H5OC6H5, and so on are examples.
Preparation of Ethers
- Alcohols are dehydrated in the following ways:
The nucleophilic bimolecular reaction forms ether when primary alcohols are treated with protic acids (H2SO4, H3PO4). This reaction is temperature dependent; alkene is a main product at 443 K, but ether is obtained as a main product at 413 K.
In elimination reaction of secondary or tertiary alcohol, alkene is formed as a main product.
Synthesis by Williamson:
When sodium alkoxide reacts with an alkyl halide, ether is produced.
When using a 1° alkyl halide, SN2 is preferred and ether is formed as a major product; however, when using a 2° or 3° alkyl halide, elimination proceeds and alkene is formed as the major product.
This process can also be used to convert phenol to ether.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Chemical Reactions of Ethers
- Cleavage of the C-O bond in reactions:
Ethers, on the other hand, are less reactive. Cleavage of the C-O bond happens at extreme circumstances.
When dialkyl ethers react with HX, they produce two alkyl halides.
Because the aryl-oxygen bond is more stable, alkyl aryl ethers react with HX to generate phenol and alkyl halide.
The reactivity order of HX is
HI > HBr > HCl
- Substitution of Electrophilic Compounds:
Electrophilic aromatic substitution occurs at the ortho (o) and para (p) positions in aryl ethers.
Friedel's reaction is as follows:
At the o and p positions, halogenation and nitration occur.
In the presence of AlCl3 (anhydrous), anisole combines with alkyl or acyl halides to produce o and p substituted products.
Also check-
