Esterification - Meaning, Definition, Examples, Uses, FAQs
Define Esterification.
Esterification Meaning: The compound is produced by a reaction between a carboxylic acid reaction and an alkyl (alkoxy) group in an acid (organic or inorganic). They have a crucial role in biology, comprising the bulk of animal fats and vegetable oils, and are one of the main classes of lipids. Aromatic esters typically have a pleasing smell; low-molecular-weight esters are commonly found in pheromones and essential oils as fragrances. In recent decades, they have become one of the most important synthetic lubricants on the commercial market and serve as excellent solvents for a broad spectrum of plastics, plasticizers, resins, and lacquers. The backbone of DNA molecules is formed by phosphodiester. The explosive properties of nitro glycerine and other nitrate esters are well known.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Define Esterification.
- Esterification Reaction Example:
- Esters: their uses
- Isomers of organic compounds
- IUPAC nomenclature
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Esterification Reaction Example:
In the process of forming the ester, a chemical reaction takes place called esterification.
A chemical reaction that results in the formation of at least one ester product occurs when an organic acid alcohol (RCOOH) is combined with an alcohol (ROH) to form an ester (RCOOR) and water. Carboxylic acid reactions and alcohol are esterified to produce an ester.
Here is an explanation of the esterification chemical reaction.
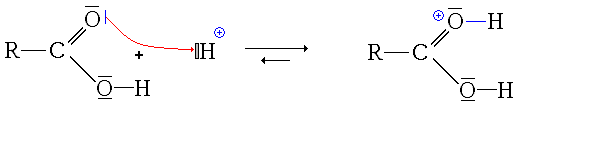
Read more :
- NCERT notes Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- NCERT solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- NCERT Exemplar Class 12 Chemistry solutions Chapter 11 Alcohols, Phenols and Ethers
Esterification reaction:
Sulphuric acid alcohol or carboxylic acid reactions can be used to form compounds with primary alcohol. A sweet smell emanates from this compound. As a result, an ester is obtained. Chemically, esterification occurs when an ester is formed.
R-C(=O)-OH + R’-OH → R-C(=O)-O-R’
Esters and their properties
As a result of the esterification reaction, an ester is named after its carboxylic acid reactions.
An enjoyable smell emanates from them.
The food and perfume industries use them extensively.
Oils and fats contain esters, which are organic compounds.
Related Topics Link |
Esters: their uses
Here are some examples of common ester applications.
Food flavourings, perfumes, and cosmetics use esters that contain fragrant compounds.
A solvent for organics.
The explosive properties of nitrogen glycerine have made it famous.
Detergents, soaps, and detergents contain surfactants.
Ester compounds are found in pheromones naturally
DNA is made of phosphate esters, which form the backbone of the molecule.
Plastics are produced using polyesters
NCERT Chemistry Notes :
Isomers of organic compounds
It is also possible to make esters from inorganic acids.
Triphenyl phosphate is one of the phosphate esters formed by phosphoric acid
Dimethyl sulphate is a sulphate ester formed by sulfuric acid
Nitrogenating of nitrate esters produces methyl nitrate, e.g.
Trimethyl borate, for example, is the result of boric acid.
In the presence of carbonic acid, a carbonate ester is formed, e.g., ethylene carbonate
An organic acid that exists as a tautomer of its esters
Phosphate esters are made up of phosphatidic acid, i.e., triethyl phosphate (P(OEt)3) and diethyl phosphate (HP(O) (O Et)2).
Stable esters are formed by inorganic acids that are unstable or elusive.
Despite never being discovered, chromic acid forms di-tert-butyl chromate
Dimethyl sulphide is formed by sulphurous acid, a rare substance
Many hundreds of alkoxides, including those made from all metals and metalloids, can be classified as esters of the hypothetical acids.
IUPAC nomenclature
Inorganic and organic acids may both exist as parent acids of esters, where the latter comes from the parent alcohol. Esters are derived from one or more complex carboxylic acid reactions are more commonly specified using the systematic IUPAC names, which are derived from naming the acids followed by "-date". Using hexyl octanoate as an example, the formula is CH3(CH2)6CO2(CH2)5CH3 and it has the trivial name hexyl caprylate. As a rule, most organic esters have the following chemical formula: RCO2R′, where R and R′ are the hydrocarbon components, respectively, of carboxylic acid reactions and alcohol. Among the many products of butanol and acetic acid (systematically ethanoic acid), butyl acetate (Butyl ethanoate) is composed of CH3CO2C4H9. In addition to Bu O Ac, CH3COOC4H9 is also common.
Also read -
Frequently Asked Questions (FAQs)
Examples of esters are methyl formate, ethyl acetate, etc.
Esterification reactions can only occur in presence of acid catalyst as well as heat. It takes lot of energy to remove the -OH from carboxylic acid, so catalyst along with heat are needed to produce required energy..
The esterification reaction has two major uses:
1. Medicines are manufactured.
2. Paint and dye manufacturing.
Esters, which contain fragrant compounds, are used to flavour foods, perfumes, and cosmetics.
Also used as Organic solvents.
A chemical reaction called esterification occurs during the formation of the ester.
An organic acid (RCOOH) and alcohol (ROH) combine to form an ester (RCOOR) and water in the presence of at least one ester product obtained from the chemical reaction. An ester is produced by esterifying carboxylic acid reactions and alcohol.
Also Read
30 Sep'24 11:38 AM
30 Sep'24 08:59 AM
21 Jul'22 03:44 PM
16 Jul'22 05:44 PM
16 Jul'22 05:33 PM
16 Jul'22 05:08 PM
16 Jul'22 05:01 PM