1. What is a Hydroxyl group?
A hydrogen atom is covalently linked to an oxygen atom to form the functional group known as the hydroxyl group. In chemical structures, the hydroxyl group is indicated by the symbol -OH  and has a negative valence charge (-1). Due to its high reactivity, the hydroxyl radical interacts with other chemical species relatively fast.
and has a negative valence charge (-1). Due to its high reactivity, the hydroxyl radical interacts with other chemical species relatively fast.
2. Name a few commercially important alcohols.
A few commercially important alcohols are methanol, ethanol, isopropyl alcohol, ethylene glycol, glycerol, etc.
3. Name a few reactions to produce alcohol.
Alcohols can be produced by reducing carbonyl compounds (Aldehydes or Ketones), by hydration of alkenes, by displacement of alkyl halides, by using Grignard and organolithium reagents, etc.
4. What are the uses of alcohol?
The uses of alcohol are as follows:
Ethanol is used as a solvent to dissolve organic compounds that are immiscible in water.
Ethanol and Methanol have been used as fuel.
Ethanol is used as an alcoholic beverage.
Alcohol is used as a common ingredient in cough syrups.
Ethanol and isopropyl alcohol are used to sanitise and sterilize to prevent microbial contamination.
A mixture of alcohols is used as a mobile phase in chromatographic analysis.
5. What is an esterification reaction?
Esterification reaction is a reaction where alcohol reacts with carboxylic acid to produce an ester which has a fruity odour.
6. How are alcohols named according to IUPAC nomenclature?
Alcohols are named by replacing the "-e" ending of the parent alkane with "-ol". The position of the hydroxyl group is indicated by a number prefix. For example, CH3-CH2-CH2-OH is named propan-1-ol.
7. How does the structure of an alcohol affect its rate of oxidation?
The rate of oxidation decreases from primary to secondary to tertiary alcohols. Primary alcohols can be oxidized to aldehydes and then to carboxylic acids, secondary alcohols to ketones, while tertiary alcohols are resistant to oxidation under normal conditions.
8. What is the significance of alcohols in green chemistry and sustainable processes?
Alcohols play a significant role in green chemistry as renewable feedstocks (e.g., bioethanol), environmentally friendly solvents, and reagents in various sustainable processes. They can be derived from biomass and often have lower toxicity compared to many petroleum-based alternatives.
9. How does the structure of an alcohol affect its ability to form coordination compounds with metals?
Alcohols can act as ligands in coordination compounds, with the oxygen atom donating a lone pair to the metal. The steric bulk around the hydroxyl group and the electron-donating or withdrawing properties of other substituents can affect the strength of this coordination.
10. How does the presence of a hydroxyl group affect the polarity of alcohols?
The hydroxyl group makes alcohols polar molecules. The electronegative oxygen atom creates a partial negative charge, while the hydrogen has a partial positive charge. This polarity leads to hydrogen bonding between alcohol molecules and affects their physical properties.
11. How does the structure of an alcohol affect its boiling point compared to alkanes?
Alcohols have higher boiling points than alkanes of similar molecular weight due to hydrogen bonding between alcohol molecules. This intermolecular force requires more energy to overcome, resulting in higher boiling points.
12. Why are small alcohols soluble in water while larger alcohols are not?
Small alcohols are soluble in water because their hydroxyl groups can form hydrogen bonds with water molecules. As the hydrocarbon chain lengthens, the non-polar portion becomes dominant, reducing water solubility.
13. How does the presence of a hydroxyl group affect the acidity of alcohols?
The hydroxyl group makes alcohols weakly acidic. They can donate a proton (H+) to strong bases, but are much less acidic than water or carboxylic acids due to the poor stability of the resulting alkoxide ion.
14. What is the difference between ethanol and methanol in terms of toxicity?
Methanol is significantly more toxic than ethanol. While the body can metabolize ethanol, methanol is converted to formaldehyde and formic acid, which can cause blindness and death even in small amounts.
15. What is the defining structural feature of an alcohol?
The defining structural feature of an alcohol is the hydroxyl group (-OH). This functional group consists of an oxygen atom covalently bonded to a hydrogen atom, and it is attached to a carbon atom in the main hydrocarbon chain or ring.
16. What is the general formula for an alcohol?
The general formula for an alcohol is R-OH, where R represents an alkyl group (a hydrocarbon chain) and -OH is the hydroxyl group.
17. What are the three classes of alcohols, and how are they distinguished?
The three classes of alcohols are primary, secondary, and tertiary. They are distinguished by the number of carbon atoms directly bonded to the carbon bearing the hydroxyl group: primary (one), secondary (two), and tertiary (three).
18. What is the difference between an alcohol and a phenol?
An alcohol has a hydroxyl group attached to an sp3 hybridized carbon in an aliphatic chain, while a phenol has a hydroxyl group directly attached to an aromatic ring. Phenols are generally more acidic than alcohols.
19. How does the position of the hydroxyl group affect the reactivity of an alcohol?
The position of the hydroxyl group affects reactivity by influencing the stability of reaction intermediates. Generally, primary alcohols are more reactive than secondary, which are more reactive than tertiary in nucleophilic substitution reactions.
20. What is meant by the term "denatured alcohol"?
Denatured alcohol is ethanol that has been mixed with toxic or unpalatable substances to make it unfit for consumption. This is done to avoid alcohol taxes on non-beverage applications while preventing misuse.
21. What is the importance of alcohols in organic synthesis?
Alcohols are versatile intermediates in organic synthesis. They can be converted to other functional groups (e.g., alkyl halides, aldehydes, ketones) and serve as nucleophiles in various reactions, making them valuable building blocks.
22. What is the difference between elimination and substitution reactions in alcohols?
In elimination reactions, alcohols lose the -OH group and a hydrogen from an adjacent carbon to form an alkene. In substitution reactions, the -OH group is replaced by another functional group without changing the carbon skeleton.
23. What is the role of alcohols in the production of esters?
Alcohols react with carboxylic acids in a condensation reaction to form esters, releasing water as a byproduct. This process, known as esterification, is important in producing flavors, fragrances, and some polymers.
24. What is the importance of alcohols in the production of polymers?
Alcohols are important in polymer production as monomers or reagents. For example, ethylene glycol is used in the production of polyester, and methanol is used in the production of formaldehyde, a precursor to many resins.
25. What is the role of alcohols in the production of biodiesel?
Alcohols, typically methanol or ethanol, react with triglycerides (fats or oils) in a process called transesterification to produce biodiesel. The alcohol breaks the triglyceride into fatty acid methyl or ethyl esters, which serve as the biodiesel fuel.
26. What is the role of alcohols in the production of ethers?
Alcohols can react with each other in the presence of an acid catalyst to form ethers. This dehydration reaction, known as the Williamson ether synthesis when using an alkoxide and an alkyl halide, is a common method for ether production.
27. What is the significance of alcohols in the food and beverage industry?
Alcohols play various roles in the food and beverage industry. Ethanol is the primary alcohol in alcoholic beverages, while other alcohols contribute to flavors and aromas. Alcohols are also used as solvents for extractions and as preservatives.
28. What is the role of alcohols in the production of halogenated compounds?
Alcohols can be converted to alkyl halides through reactions with hydrogen halides or phosphorus trihalides. This conversion is important in organic synthesis as it transforms the poorly leaving hydroxyl group into a good leaving group.
29. What is the role of alcohols in the production of fuel additives?
Alcohols, particularly ethanol and methanol, are important fuel additives. They increase the octane rating of gasoline, reduce carbon monoxide emissions, and can be used as alternative fuels. Ethanol is commonly blended with gasoline to produce gasohol.
30. What is the role of alcohols in the production of plasticizers?
Alcohols are important precursors in the production of plasticizers. They react with phthalic anhydride or other acids to form esters that serve as plasticizers, improving the flexibility and durability of plastics.
31. What is the significance of hydrogen bonding in alcohols?
Hydrogen bonding in alcohols is significant because it affects many physical properties, including boiling point, solubility, and viscosity. It occurs between the partially positive hydrogen of one alcohol molecule and the partially negative oxygen of another.
32. How does the structure of an alcohol influence its melting point?
The melting point of alcohols is influenced by molecular weight and the strength of intermolecular forces. Straight-chain alcohols tend to have higher melting points than branched isomers due to more efficient packing in the solid state.
33. How does the hydroxyl group affect the infrared (IR) spectrum of alcohols?
The hydroxyl group produces a characteristic broad absorption band in the IR spectrum around 3200-3600 cm-1 due to O-H stretching. This band is broader than the sharp O-H peak of free alcohols due to hydrogen bonding.
34. How does hydrogen bonding affect the viscosity of alcohols?
Hydrogen bonding increases the viscosity of alcohols compared to hydrocarbons of similar molecular weight. The intermolecular attractions cause the molecules to resist flow, leading to higher viscosity.
35. How does the structure of an alcohol affect its reactivity in elimination reactions?
The tendency for elimination increases from primary to secondary to tertiary alcohols. This is due to the increasing stability of the carbocation intermediate formed during the reaction.
36. What is the significance of the hydroxyl group in biological systems?
The hydroxyl group is crucial in biological systems for its ability to form hydrogen bonds. This property is important in protein folding, DNA base pairing, and the solubility of many biomolecules in aqueous environments.
37. How does the presence of multiple hydroxyl groups affect the properties of a compound?
Compounds with multiple hydroxyl groups, such as glycerol or sugars, have increased hydrophilicity and hydrogen bonding capabilities. This leads to higher boiling points, increased water solubility, and unique chemical behaviors.
38. What is the mechanism of the reaction between an alcohol and a strong base?
When an alcohol reacts with a strong base, the base removes the proton from the hydroxyl group, forming an alkoxide ion. This alkoxide ion is a strong nucleophile and can participate in various reactions.
39. How does the structure of an alcohol affect its ability to act as a nucleophile?
The nucleophilicity of alcohols decreases from primary to secondary to tertiary due to steric hindrance. Primary alcohols, having less steric bulk around the oxygen, are generally better nucleophiles than secondary or tertiary alcohols.
40. What is the difference between anhydrous and hydrous alcohols?
Anhydrous alcohols contain no water, while hydrous alcohols contain some water. The presence of water can significantly affect the reactivity and properties of the alcohol, particularly in reactions sensitive to moisture.
41. How does the structure of an alcohol affect its ability to form azeotropes?
The tendency to form azeotropes (constant boiling mixtures) with water depends on the alcohol's structure. Lower molecular weight alcohols like ethanol form azeotropes more readily than higher molecular weight alcohols due to stronger hydrogen bonding with water.
42. How does the presence of a hydroxyl group affect the NMR spectrum of a molecule?
In proton NMR, the hydroxyl proton typically appears as a broad singlet due to hydrogen bonding and rapid exchange. In carbon-13 NMR, the carbon attached to the hydroxyl group is usually shifted downfield compared to alkyl carbons.
43. How does the structure of an alcohol affect its rate of esterification?
The rate of esterification generally decreases from primary to secondary to tertiary alcohols due to increasing steric hindrance. Primary alcohols react faster as they have less steric bulk around the reactive hydroxyl group.
44. What is the significance of alcohols in the pharmaceutical industry?
Alcohols are important in pharmaceuticals as solvents, reagents, and as components of many drugs. The hydroxyl group can serve as a pharmacophore, influencing a drug's solubility, metabolism, and interaction with biological targets.
45. How does the presence of a hydroxyl group affect the UV-Vis spectrum of a molecule?
The hydroxyl group itself doesn't typically absorb in the UV-Vis region, but it can influence the spectrum through its electron-donating properties when conjugated with other functional groups, potentially causing a bathochromic shift (shift to longer wavelengths).
46. What is the difference between a hemiacetal and an acetal, and how are alcohols involved in their formation?
Hemiacetals form when one molecule of an alcohol adds to an aldehyde. Acetals form when a second alcohol molecule reacts with the hemiacetal. Alcohols are reactants in both cases, with the hydroxyl group adding to the carbonyl carbon.
47. How does the structure of an alcohol affect its ability to act as a leaving group in nucleophilic substitution reactions?
Alcohols are poor leaving groups in nucleophilic substitution reactions due to the strong C-O bond. To improve their leaving ability, they are often converted to better leaving groups like alkyl halides or tosylates.
48. How does the presence of a hydroxyl group affect the dipole moment of a molecule?
The hydroxyl group increases the dipole moment of a molecule due to the electronegativity difference between oxygen and hydrogen. The magnitude of the dipole moment depends on the overall molecular structure and the position of the hydroxyl group.
49. How does the structure of an alcohol affect its ability to form hydrogen bonds with itself versus with water?
The ability of an alcohol to form hydrogen bonds with itself versus water depends on the balance between the polar hydroxyl group and the non-polar hydrocarbon portion. Short-chain alcohols form more hydrogen bonds with water, while longer-chain alcohols tend to associate more with themselves.
50. How does the presence of a hydroxyl group affect the reactivity of adjacent functional groups?
The hydroxyl group can influence adjacent functional groups through inductive and resonance effects. It can activate or deactivate aromatic rings, affect the acidity of nearby protons, and participate in intramolecular hydrogen bonding, altering reactivity patterns.
51. What is the importance of alcohols in the production of perfumes and fragrances?
Alcohols are crucial in perfumery as solvents and fixatives. They also contribute directly to fragrances, with many naturally occurring alcohols having pleasant scents. Additionally, alcohols can react to form esters, which are often key fragrance components.
52. How does the structure of an alcohol affect its ability to act as a hydrogen bond donor versus acceptor?
All alcohols can act as both hydrogen bond donors (via the H in OH) and acceptors (via the O in OH). However, the effectiveness can vary with structure. Sterically hindered alcohols may be poorer donors, while electronegative substituents can enhance the acceptor ability of the oxygen.
53. How does the presence of a hydroxyl group affect the reactivity of a molecule in free radical reactions?
The hydroxyl group can participate in free radical reactions, particularly through hydrogen abstraction. The strength of the O-H bond and the stability of the resulting radical influence the molecule's reactivity in these processes.
54. How does the presence of a hydroxyl group affect the crystalline structure of a solid alcohol?
The hydroxyl group significantly influences the crystalline structure of solid alcohols through hydrogen bonding. This can lead to the formation of complex crystal lattices, affecting properties such as melting point, solubility, and polymorphism.
55. What is the importance of alcohols in the field of surface chemistry and interfacial phenomena?
Alcohols are important in surface chemistry due to their amphiphilic nature. They can act as surfactants, reducing surface tension and stabilizing emulsions. This property is crucial in various applications, from cleaning products to oil recovery and nanoparticle synthesis.
![]() ) is substituted for the hydrogen atom of aliphatic carbon in a particular class of organic molecules known as alcohols. So an alcohol molecule has two components, one with an alkyl group and the other with a hydroxyl group. R-OH
) is substituted for the hydrogen atom of aliphatic carbon in a particular class of organic molecules known as alcohols. So an alcohol molecule has two components, one with an alkyl group and the other with a hydroxyl group. R-OH ![]() , where R is the alkyl group, is used to represent it. Alkanes and alcohols have the same basic chemical formula, but alcohols have a functional group called the hydroxyl group, which has the chemical formula -OH
, where R is the alkyl group, is used to represent it. Alkanes and alcohols have the same basic chemical formula, but alcohols have a functional group called the hydroxyl group, which has the chemical formula -OH ![]() . The most widely used alcohol is ethanol, which is also used as fuel (gasoline), a preservative for biological samples, a solvent for paints, and used in medicine.
. The most widely used alcohol is ethanol, which is also used as fuel (gasoline), a preservative for biological samples, a solvent for paints, and used in medicine.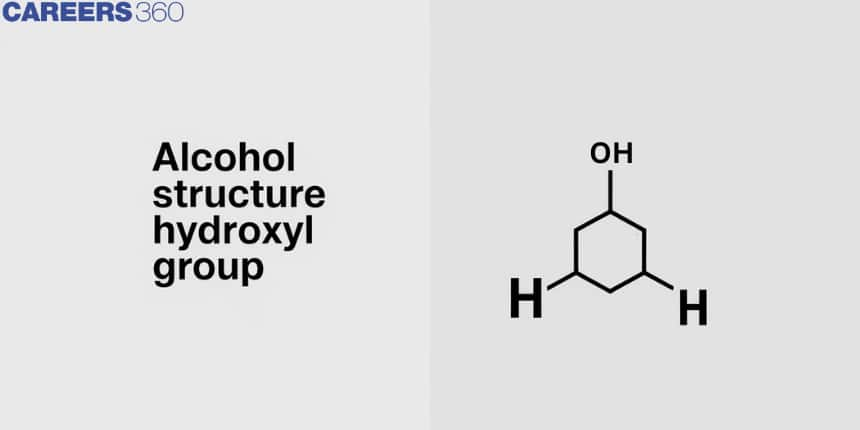
![]() . The parent chain is numbered at the end closest to the -OH
. The parent chain is numbered at the end closest to the -OH ![]() .
.![]() , where n = 1, 2, 3.
, where n = 1, 2, 3.![]() bond angle in alcohols is typically greater than the 104.5° H-O-H
bond angle in alcohols is typically greater than the 104.5° H-O-H ![]() bond angle in a water molecule. The 108.9° bond angle in methanol, for instance, demonstrates the impact of the methyl group, which is heavier than the hydrogen atom in water.
bond angle in a water molecule. The 108.9° bond angle in methanol, for instance, demonstrates the impact of the methyl group, which is heavier than the hydrogen atom in water.![]() and has a negative valence charge (-1). Due to its high reactivity, the hydroxyl radical interacts with other chemical species relatively fast.
and has a negative valence charge (-1). Due to its high reactivity, the hydroxyl radical interacts with other chemical species relatively fast.