Alcohols, Phenols and Ethers
Detergents, antiseptics, and scents are all made up of three basic compounds: Alcohols, Phenols, and Ethers.
You've learned that replacing one or more hydrogen atoms in a hydrocarbon with another atom or a group of atoms produces a totally new chemical with entirely different properties and applications. When a hydrogen atom in an aliphatic or aromatic hydrocarbon is replaced by a –OH group, alcohols and phenols are produced. These types of chemicals have a wide range of applications in both business and everyday life.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs
- Important Topics
- Overview Of The Chapter
- Importance Of Alcohols, Phenols, And Ethers In Class 12:
Have you ever noted that ordinary spirit, which is used to polish wooden furniture, is mostly a hydroxyl group-containing molecule called ethanol? Compounds containing –OH groups are found in the sugar we eat, the cotton we wear, and the paper we write on. Consider life without paper: no notebooks, books, newspapers, cash notes, checks, or certificates, to name a few examples. Beautiful images and engaging tales would vanish from our lives, as would publications. It'd have been a very different planet.
A phenol has –OH group(s) directly attached to a carbon atom(s) of an aromatic system, whereas alcohol contains one or more hydroxyl (OH) group(s) directly attached to a carbon atom(s) of an aliphatic system (CH3OH) (C6H5OH).
Another class of compounds known as 'ethers' is formed when a hydrogen atom in a hydrocarbon is replaced by an alkoxy or aryloxy group (R–O/Ar–O). For example, CH3OCH3 (dimethyl ether).
Important Topics
Preparation Of Alcohols:
Alcohols are compounds that have attached one or more (-OH) hydroxyl groups to them. The alcohols do not occur in the free form in nature. They are obtained from flowers, leaves, and stems of plants. The Preparation Of Alcohols includes many methods such as the hydrolysis of halides, hydration of alkenes, Grignard reagents, etc
Physical And Chemical Properties Of Alcohols:
Physically, alcohols have higher boiling and melting points than alkanes and alkenes of similar molecular weight due to the presence of a strong hydrogen bond among them. Chemically, alcohols undergo acylation, whereby an acyl group is introduced into the molecule, and oxidation, whereby alcohol is oxidized into aldehydes, ketones, or carboxylic acids. There are many other Physical And Chemical Properties Of Alcohols.
Preparation Of Phenols:
The preparation of phenol in most cases involves the hydroxylation of an aromatic compound, usually benzene. Phenol is manufactured from the hydrocarbon, cumene. Cumene (isopropylbenzene) is oxidized in the presence of air to cumene hydroperoxide.
Physical And Chemical Properties Of Phenols:
Phenols are colorless liquids or white crystalline solids. The boiling points of phenols are much higher than the corresponding aromatic hydrocarbons and the haloarenes. Chemically, Phenols contain both a hydroxyl group and an aromatic ring; therefore, they are highly prone to various chemical reactions. In the Reimer-Tiemann reaction, phenols form ortho-hydroxybenzaldehyde upon formylation with chloroform and base. There are other many Physical And Chemical Properties Of Phenols.
Williamson's Ether Synthesis:
Williamson's Ether Synthesis is an old method in organic chemistry for the synthesis of ethers. The reaction involves the nucleophilic substitution by an alkoxide ion, RO-, by a primary alkyl halide $\mathrm{R}^{\prime}-\mathrm{X}$, to form an ether, R-O-R.
Overview Of The Chapter
Classification Of Alcohols And Phenols
Alcohols and phenols are classed as monohydric, dihydric, trihydric, or polyhydric depending on how many hydroxyl groups they have in their molecules: one, two, three, or many, respectively
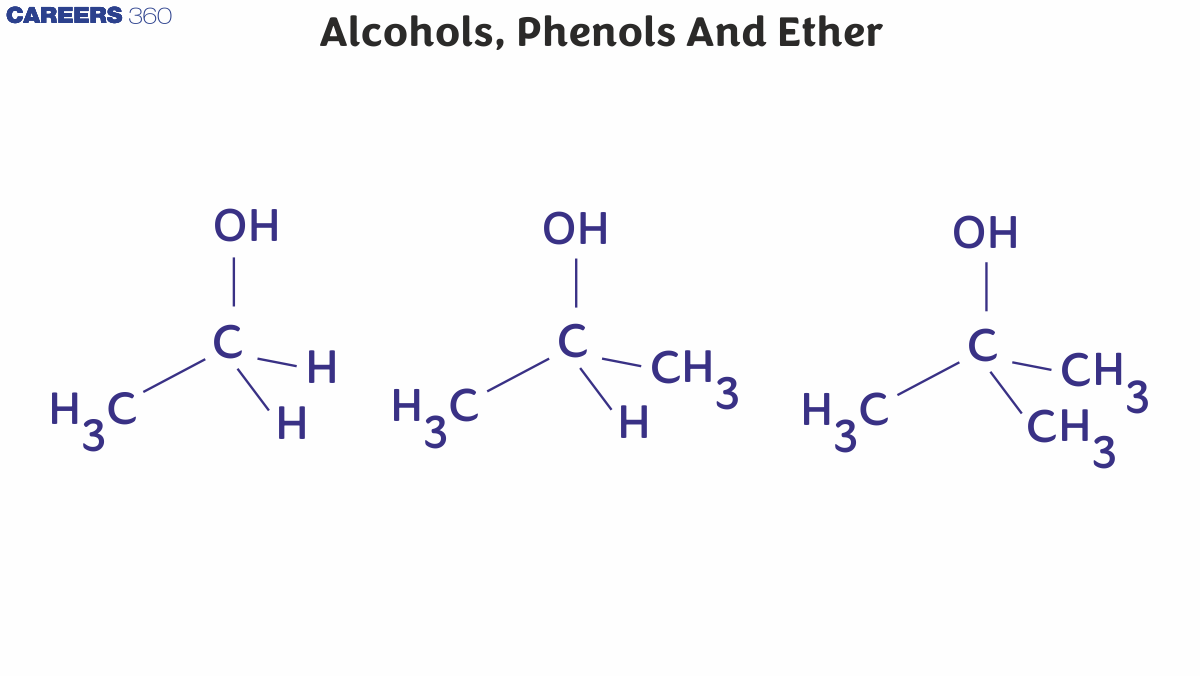
Alcohols with the OH group connected to a primary, secondary, or tertiary carbon atom are known as primary (1°), secondary (2°), and tertiary (3°) alcohols.
Allylic alcohol is made up of an -OH group attached to an sp3 Hybridised carbon next to a carbon-carbon double bond, whereas benzylic alcohol is made up of an -OH group attached to an sp3 hybridized carbon close to an aromatic ring. Alcohols having -OH group bonded to carbon-carbon double bond is called vinylic alcohol
Structure Of Alcohols And Phenols
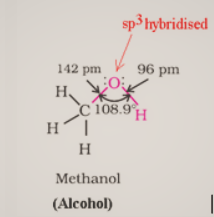
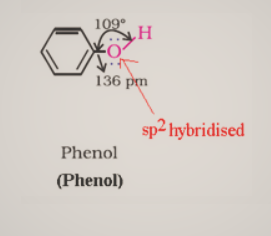
Alcohols have sp3 hybridized oxygen atoms and hybrid atomic orbitals in a tetrahedral configuration. Due to lone pair repulsion, this angle for of methyl alcohol is (C – O – H) 108.9°.The -OH group in phenols is connected to sp2 hybridized carbon, giving the C – O bond a partial double bond nature.
Preparation Of Alcohols
From alkenes
This reaction is in accordance with markovnikov's rule, through acid-catalyzed hydration.
CH3CH=CH2 + H2O → CH3-CH(OH) - CH3
By hydroboration-oxidation
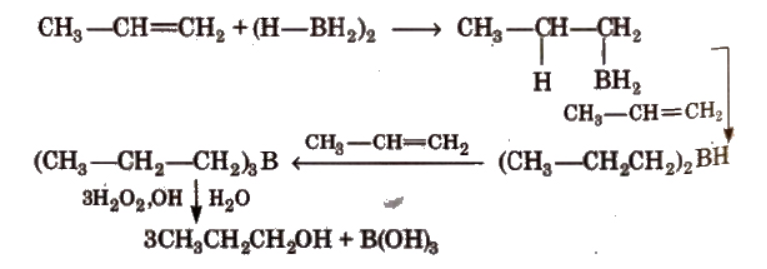
By reduction of aldehydes and ketones
RCHO + H2 → R CH2OH
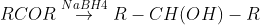
By reduction of carboxylic acids
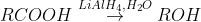
From Grignard reagents
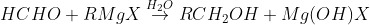
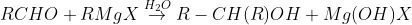
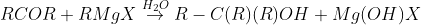
Physical Properties Of Alcohols And Phenols
Lower alcohols are colorless liquids, C5–C11 alcohols are oily liquids, and C12 and higher alcohols are waxy solids. Alcohols are miscible with water because their hydroxyl groups can form H-bonds with water. With increasing molecular mass, solubility decreases. Because polar molecules have intermolecular hydrogen bonding, the boiling points of alkanes are greater than expected.
These are colorless liquids or crystalline solids that turn coloured over time due to gradual oxidation in the presence of air. Carboxylic acids are another name for phenol. Phenols establish intermolecular H-bonds with other phenol molecules and with water due to the presence of a polar -OH bond.
Ether:
Ether is a pleasant-smelling colourless volatile liquid that is highly flammable. It is used as an anesthetic and as a solvent or intermediate in industrial processes.
Preparation Of Ether
Dehydration of alcohols
CH3CH2OH+ H2SO4,443 K→ CH2= CH2
CH3CH2OH + H2SO4,413 K→ C2H5OC2H5
Williamson synthesis
R-X + R-O-Na yields→ R-O-R + NaX
Physical Properties Of Ethers
Since ethers' C-O bonds are polar, they have a net dipole moment. Their boiling points are equivalent to alkanes with similar molecular weights, although they are lower than alcohols. It's because ethers don't have H-bonding. Ether miscibility with water is similar to that of alcohols of the same molar mass. It's because others, like alcohol, can make H-bonds with water.
Importance Of Alcohols, Phenols, And Ethers In Class 12:
Alcohol and ether are classes of organic compounds that find wide usage in industries as well as for domestic purposes. Alcohol is formed when a saturated carbon atom is bonded to a hydroxyl (-OH) group. Ether is formed when an oxygen atom is connected to two alkyl or aryl groups.
Although, in the JEE Examination, there are just one to two questions about alcohol, Phenols, and Ethers.
However, it is still very important.
The main concepts include the classification of alcohol and ether, preparation and important reactions. Alcohol and Ether is an important topic included in the JEE syllabus for organic chemistry. Questions based on their reactions and methods of preparation are asked almost every year in JEE.
Frequently Asked Questions (FAQs)
Alcohols usually feature the hydroxyl group attached to aliphatic hydrocarbons. Phenols usually contain aromatic hydrocarbons. In comparison to phenol, alcohols are known to be less acidic. Phenols are relatively more acidic in nature and should, therefore, be diluted before usage.
The basic components used to make detergents, antiseptics, and perfumes are alcohols, phenols, and ethers, respectively.
Test to distinguish between alcohol and phenols
1. Litmus test: Phenols turn blue litmus red and alcohols have no effect.
2. Ferric chloride test: Phenols give blue, green, and violet colour while alcohol gives no change in colour.
3. Bromine water test: Phenols gives white precipitates while alcohol gives no precipitates.
A hydroxyl group is attached to a carbon atom of alcohols. —OH is attached to a benzene ring in phenols. (This class's "parent" molecule ule is also called phenol: PhOH or C6H5OH.) The ether functional group is formed when two carbon groups are connected to oxygen by single bonds.
Ortho-nitrophenol has a lower boiling point due to the formation of intramolecular H-bonding whereas p-nitrophenol forms intermolecular H-bonding.
Also Read
19 Dec'24 11:18 PM
30 Sep'24 11:38 AM
30 Sep'24 08:59 AM
21 Jul'22 03:44 PM
16 Jul'22 05:44 PM
16 Jul'22 05:33 PM
16 Jul'22 05:08 PM