Methods of Preparation of Aldehydes and Ketones
Aldehydes and ketones can be prepared using various methods. Aldehydes are commonly synthesized through the oxidation of primary alcohols, controlled oxidation of alkenes, or by using reagents like PCC. Ketones, on the other hand, can be prepared by oxidizing secondary alcohols, through the hydration of alkynes, or via Friedel-Crafts acylation. Methods of preparation of aldehydes and ketones highlight the versatility of organic reactions in synthesizing these compounds.
Reactions of Aldehydes and Ketones
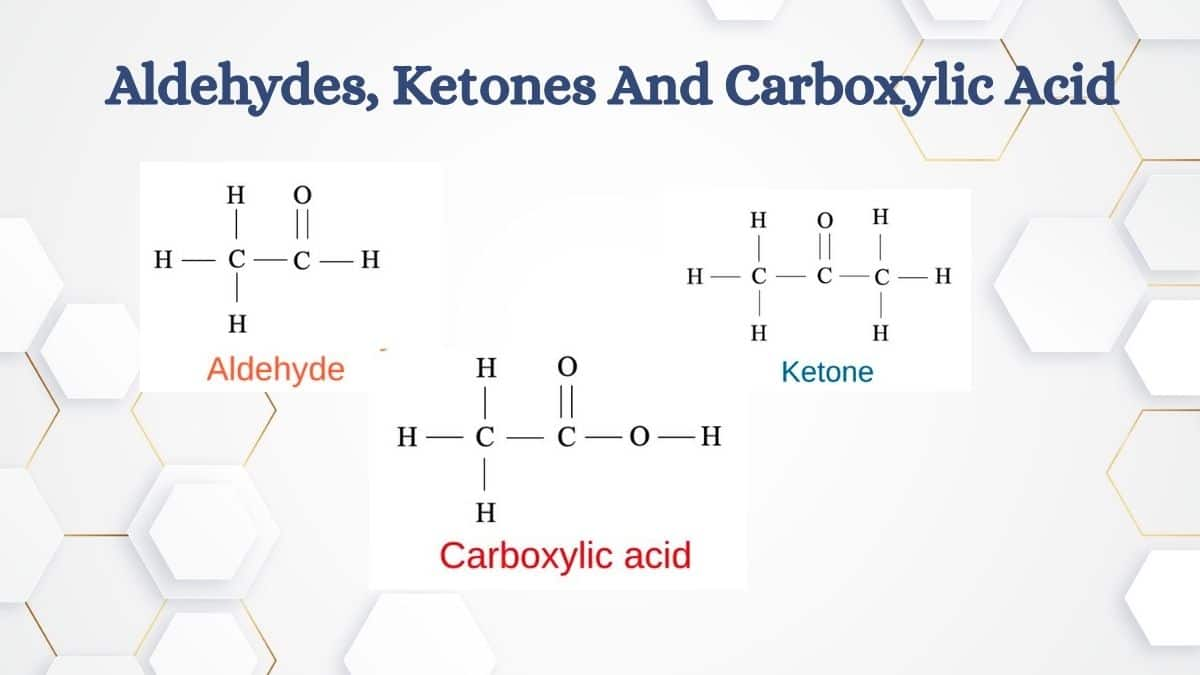
Aldehydes and ketones exhibit a wide range of chemical reactions due to the presence of the carbonyl group (C=O). They undergo nucleophilic addition reactions such as the formation of cyanohydrins and hemiacetals. Aldehydes, being more reactive, also participate in oxidation reactions to form carboxylic acids and can reduce to primary alcohols. Ketones undergo similar reduction reactions to yield secondary alcohols. Special reactions like aldol condensation and Cannizzaro reaction further illustrate their reactivity.
Carboxylic Acids
Carboxylic acids are organic compounds containing the carboxyl group (−COOH). They are generally prepared through the oxidation of primary alcohols or aldehydes, and hydrolysis of nitriles or esters. Carboxylic acids exhibit unique properties such as hydrogen bonding, making them highly polar with relatively high boiling points. They participate in reactions like esterification, decarboxylation, and reduction to alcohols, showcasing their versatility in organic synthesis.