Chemical Thermodynamics - Notes, Topics, Books, FAQs
Edited By Team Careers360 | Updated on Dec 19, 2024 09:46 PM IST
Thermodynamics is the branch of science which describes the principles governing energy transformations and their impact on matter. From powering engines to understanding the behaviour of natural systems, thermodynamics provides a framework for analyzing how energy is transferred, conserved, and utilized in various forms. Whether it's the heat released during combustion, the work done by a steam engine, or the energy conversion in living organisms, the study of thermodynamics is crucial for advancing technology and deepening our understanding of the universe. In this article, we will discuss the foundational concepts, laws, and applications of thermodynamics, emphasizing its relevance in both science and everyday life.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
This Story also Contains
- Important Topics- Chemical Thermodynamics
- Overview of the Chapter
- How to prepare for Chemical Thermodynamics?
- Application of Thermodynamics in Daily Life
- Prescribed Books for the Chemical Thermodynamics
Important Topics- Chemical Thermodynamics
Introduction to Thermodynamics
Thermodynamics is the study of energy transformations in physical and chemical processes, using terms like system, surroundings, state variables, and thermodynamic equilibrium. Understanding basic terminologies is important to solve numerical questions of thermodynamics.
Thermodynamic Properties
Thermodynamic properties are categorized into intensive properties and extensive properties. Intensive properties are one which are independent of the system size such as temperature and extensive properties are dependent on system size such as volume.
State Functions
State functions like enthalpy, entropy, and internal energy, depend only on the initial and final states of a system and not on the path taken to reach them.
Reversible, Irreversible, and Polytropic Processes
A reversible process occurs infinitesimally slowly, allowing the system to remain in equilibrium, while irreversible processes proceed rapidly and dissipate energy. Polytropic processes follow the equation PVn=constant, where n defines the process type.
Zeroth Law of Thermodynamics
The Zeroth Law of thermodynamics is related to the thermal equilibrium which states that if two systems are in equilibrium with a third, they are in equilibrium with each other.
Introduction to Heat, Internal Energy, and Work
Heat is energy transferred due to temperature difference, internal energy is the total energy within a system, and work is energy transferred when a force acts over a distance. Detailed study of Heat, Internal energy and work is important to solve numerical problems in thermodynamics.
First Law of Thermodynamics
The First Law of thermodynamics states that energy cannot be created or destroyed, only transformed. Mathematically, ΔU=Q−W, where ΔU is the change in internal energy, Q is heat, and W is work done.
Adiabatic Process
In an adiabatic process, no heat is exchanged with surroundings, and changes in internal energy are equal to the work done on or by the system.
Graphical Comparison of Thermodynamic Processes
Thermodynamic processes like isothermal, adiabatic, isobaric, and isochoric can be represented on PV and TS diagrams which highlights the differences in work done and heat exchange. Understanding graphical comparison of thermodynamic processes helps in solving numerical problems.
Thermochemistry
Thermochemistry is the branch of thermodynamics which deals with energy changes in chemical reactions, focusing on heat exchange at constant pressure or volume.
Enthalpy Change
Enthalpy (H) is the heat change in a process at constant pressure. Enthalpy changes include fusion, vaporization, and reaction enthalpies.
Heat Capacity: Relationship Between Cp and Cv
Cp is heat capacity at constant pressure, and Cv is at constant volume. The relationship between Cp and Cv is Cp−Cv=R for an ideal gas.
Standard Enthalpy of Formation, Combustion, and Bond Dissociation
Standard Enthalpy Of Formation, Combustion And Bond Dissociation quantify energy changes during compound formation, fuel combustion, or bond breaking, respectively, under standard conditions.
Lattice Enthalpy, Hydration Enthalpy, and Enthalpy of Solution
Lattice enthalpy measures the energy required to separate ions in a solid. Hydration enthalpy is the energy released when ions dissolve in water, and solution enthalpy combines both. Concepts like Lattice Enthalpy, Hydration Enthalpy And Enthalpy Of Solution are important to understand to solve various conceptual questions.
Enthalpy of Neutralization of Strong Acid and Base
The enthalpy of neutralization of strong acid and base is the heat change when one mole of water forms from the reaction between a strong acid and a strong base.
Ionization and Electron Gain Enthalpy
Ionization enthalpy is the energy required to remove an electron from an atom, while electron gain enthalpy is the energy change when an electron is added.
Resonance Energy
Resonance energy is the energy difference between the actual molecule and the most stable resonance structure, stabilizing molecules.
Kirchhoff’s Equation
Kirchhoff’s Equation relates the enthalpy change of a chemical reaction to the temperature at which it occurs. Kirchhoff’s Equation accounts for the variation in heat capacities of reactants and products with temperature, allowing the calculation of enthalpy change (ΔH) at one temperature from its value at another.
Hess’s Law
Hess’s Law states that the total enthalpy change in a reaction is the same regardless of the pathway, allowing calculation of unknown enthalpies.
Born-Haber Cycle
The Born-Haber Cycle is a thermochemical cycle that provides a step-by-step representation of the energy changes involved in the formation of an ionic compound from its constituent elements. Born-Haber Cycle is particularly useful for calculating the lattice enthalpy which is a key parameter for ionic solids.
| Also read, |
Overview of the Chapter
A system is something in which observations related to heat and work are done. Other than the system, everything else existing is known as the surroundings. In thermodynamics, first you need to understand various kinds of important terms as follows:
- Open System: It is the system, where the exchange of matter and energy takes place between the system and surroundings.
- Closed System: It is the system, in which exchange of matter does not take place but the exchange of energy is possible between the system and surroundings.
- Isolated System: In this system, no exchange of matter and energy is possible between the system and the surroundings.
- Adiabatic process: It is the process, in which transfer of energy does not take between the system and the surroundings.
- Intensive properties: These are those properties of the system which do not depend upon the quantity or size of the system. Common examples include temperature, density, pressure, etc.
- Extensive properties: These are those properties of the system which depends upon the quantity or size of the system. Some examples include mass, volume, internal energy, etc.
- Internal Energy: It is the total energy of the system. Energy can be of any type like mechanical, electrical, etc thus the sum of all these energies is known as the internal energy.
Fist Law of Thermodynamics
This law simply states that "energy of an isolated system is constant". In other words, energy can neither be created nor be destroyed. Mathematically, it can be expressed as follows:
Work
In thermodynamics, work is defined as the force that we applied on the system to change its volume form Vi to Vf.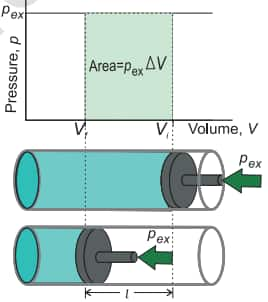
Mathematically, work can be described as follows:
Now, if the pressure is not constant but is changing infinitesimally, then work is given as follows,
Heat Capacity
When heat is applied to any system, the temperature of the system rises. But if the same amount of heat energy is given to different systems, then these systems rise with different temperatures. This variation of temperatures in different systems is only because of coefficient or heat capacity. This heat capacity is denoted by C. Thus, mathematically, heat capacity can be expressed as follows:
- Relationship between Cp and Cv for an ideal gas:
At constant volume, the equation of heat can be written as:
And at constant pressure, the equation of heat can be written as :
After the derivation, we have found the relation between Cp and Cv as follows:
NEET Highest Scoring Chapters & Topics
This ebook serves as a valuable study guide for NEET exams, specifically designed to assist students in light of recent changes and the removal of certain topics from the NEET exam.
Download EBookEnthalpies for different types of reactions
There are various kinds of enthalpies for various kinds of reactions. These are mentioned below:
- Standard enthalpy of combustion(
- Enthalpy of atomization(
- Enthalpy of solution(
- Lattice enthalpy: This is the enthalpy change when one mole of an ionic compound dissociates into its ions in the gaseous state.
Spontaneity: In simple words, a spontaneous process is a process which occurs on its own. This process might be too fast or too slow, but it does not require any external agency to occur. It is always irreversible and can only be reversed by external things. For example, the reaction of hydrogen and oxygen is very slow at normal conditions but still, it is a spontaneous reaction as it is occurring on its own.
Gibbs energy and spontaneity
Gibbs energy is an extensive property and a state function. It gives us information about the spontaneity of any reaction. It collective and takes into account both the enthalpy and the entropy. Mathematically, it can be formulated as follows:
For determining the spontaneity of any process, two cases arise for Gibbs energy as follows:
- If
- If
How to prepare for Chemical Thermodynamics?
This chapter is a part of Physical chemistry. This chapter is one of the most important chapters of the complete chemistry syllabus. Its concepts, laws, numerical and graphs all are important both for the basic foundation of chemistry and for scoring good marks in the examination.
Before reading this chapter, first, you must have the basic knowledge of the mole concept.
You must observe carefully about the enthalpies, heat capacities, entropy, etc.
Rest this chapter is very simple, just be regular and be consistent in your numerical practice.
Application of Thermodynamics in Daily Life
Thermodynamics and its laws are immensely used in our daily life and it is very interesting to understand these processes. Some of them are mentioned below:
- The ice cubes in a drink absorb heat from it and make it cold and itself melt with time gradually. This flow of heat occurs according to the first and second law of thermodynamics.

- The air conditioners in our homes also work on the principles of thermodynamics. It absorbs heat from the room and makes it cool. It throws this heat to the outside atmosphere, thus the backside of air conditioners are always warm.

- The combustion of gasoline provides the energy to the car engines, due to which we are able to travel to different places.

NCERT Exemplar Solutions Subject wise link:
- NCERT exemplar solutions for class 11 Physics.
- NCERT exemplar solutions for class 11 Chemistry.
- NCERT exemplar solutions for class 11 Mathematics.
- NCERT exemplar solutions for class 11 Biology.
Prescribed Books for the Chemical Thermodynamics
First, you must finish the class XI NCERT book and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow R.C. Mukherjee and O.P. Tandon. You must definitely solve the previous year papers. Meanwhile, in the preparation, you must continuously write the mock tests for the depth of knowledge. Our platform will help you to provide with the variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Frequently Asked Questions (FAQs)
1. What is thermodynamics?
Thermodynamics is the branch of science that deals with the study of energy changes, particularly heat and work, during physical and chemical processes.
2. What is the first law of thermodynamics?
The first law of thermodynamics states that energy can neither be created nor destroyed, only transformed or transferred. Mathematically, it is expressed as: ΔU=q+w
Where ΔU is the change in internal energy, q is heat, and w is work.
3. What is internal energy (U)?
Internal energy is the total energy contained within a system, including the kinetic and potential energy of all particles in the system. It changes when the system exchanges heat or work with its surroundings.
4. What is the difference between heat and work?
Heat (q) is the transfer of energy due to a temperature difference, while work (w) is the energy transfer resulting from a force acting over a distance, such as pressure-volume work in a gas.
5. What is the significance of specific heat capacity?
Specific heat capacity is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. It helps in understanding how substances absorb and transfer heat.
Also Read
Entropy Change
19 Feb'25 12:52 PM
Graphical Comparison of Thermodynamic Processes
19 Feb'25 10:40 AM
Zeroth Law Of Thermodynamics
19 Feb'25 10:36 AM
State Functions
19 Feb'25 10:35 AM
Tautomerism
19 Feb'25 10:16 AM
Second Law of Thermodynamics
18 Feb'25 11:45 PM
Spontaneity in Thermodynamics
18 Feb'25 11:43 PM
Born Habers Cycle
18 Feb'25 11:40 PM
Hess’s Law
18 Feb'25 11:38 PM
Resonance Energy
18 Feb'25 11:36 PM
Articles
May 02, 2025
Apr 26, 2025
News and Notifications
Back to top

