Alkanes - Definition. Properties, Formulas, FAQs
A series of compounds containing Hydrogen and carbon atoms with a single covalent bond constitutes an alkane series. There are compounds in this category comprised of carbon and hydrogen atoms linked by one covalent bond. It also appears to contain a Homologous Series of compounds with a molecular general formula of alkane of CnH2n+2.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Hydrocarbons
- Alkanes: Physical Properties
- Formula and Alkane Structure of Alkanes
- Chemistry of Alkane Formulas
- Alkane Formula with Branched Chains
- Groups and Alkyls
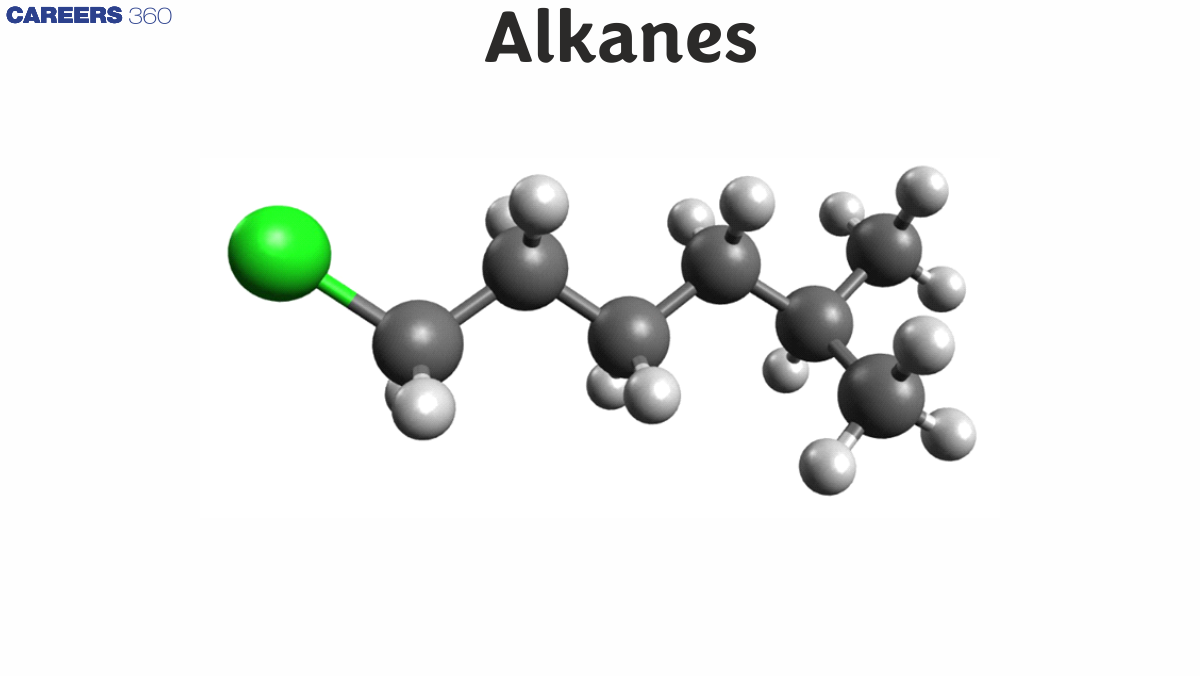
All alkanes can be divided into two groups based on their structure. Carbon and hydrogen are the only elements in them. One bond form between each hydrogen atom and four bonds between each carbon atom.
Hydrocarbons
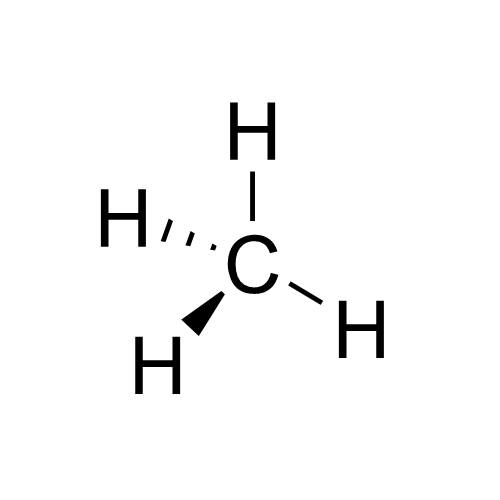
Its molecular formula is CH4 and it comes from the simple alkane methane ethane propane-butane. Alkane structural formula is for a compound with only one covalent bond
Also read :
Methane
An additional carbon atom is attached to another carbon atom via a covalent bond in a long-chain alkane molecule. A total of four single covalent bonds are formed between each atom and enough hydrogen atoms. Oxana is a long-chained hydrocarbon. Alkanes with eight carbons have the molecular formula C8H18.
Alkanes: Physical Properties
1.Alkane Solubility
Generally speaking, alkanes are non-polar molecules due to the fact that carbon and hydrogen have little difference in Electronegativity, and they have a covalent bond between them.
A general rule of thumb is that polar molecules are soluble in polar solvents, whereas nonpolar molecules are soluble in nonpolar ones. The nature of alkanes, therefore, is hydrophobic, meaning they are insoluble in water.
They are however soluble in organic solvents since overcoming existing Van Der Waals Forces and generating new ones takes about the same amount of energy.
2. Alkanes and their boiling points
With increasing molecular weight, alkanes have a higher boiling point,
A comparison of the boiling points of straight-chain alkanes and their structural isomers reveals the former has a higher boiling point.
3.The melting points of alkanes
An alkane's melting point increases as its molecular weight increases, i.e., it is proportional to its boiling point.
There is a strong attraction between alkanes that makes it hard to overcome them due to their solid nature.
The melting point of even-numbered alkanes tends to be higher than that of odd-numbered alkanes because the even-numbered alkanes form well-organized crystal alkane structures in solid-state, making them difficult to seep out.
Formula and Alkane Structure of Alkanes
Condensed forms of alkane structural formulas are available. Pentane is a chemical compound containing three CH2 methylene groups in the middle of the chain.The structural formula can be written by grouping them together. The first five unbranched alkane formulas are listed below.
Name | The molecular formula of alkane | The condensed structural formula of alkane |
methane | CH4 | CH4 |
ethane | C2H6 | CH3CH3 |
propane | C3H8 | CH3CH2CH3 |
butane | C4H10 | CH3(CH2)2CH3 |
Chemistry of Alkane Formulas
There is a wide variety of information to be found in organic compound formulas. The atomic numbers for molecules of a compound can be calculated by looking at a molecular formula, like the one for octane. Alkanes have various properties, including chemical, physical, and physicochemical, and the Molecular Formula of C8H18 can apply to many of them.
Each of these compounds has a specific alkane structure formula showing the atoms in the molecule arranged in a certain order. Isomerization refers to the process of changing the alkane structure of organic compounds with the same molecular formula.
There are many organic compounds that can be converted into alkanes. The functional groups of many organic molecules are alkane groups substituted for the hydrogen atom of the basic molecule. Because of these factors, alkanes are sometimes named for organic compounds.
Alkane Formula with Branched Chains
Alkanes contain carbon atoms that form straight chains, branched chains and rings like other organic compounds. A straight-chain alkane, a branched-chain alkane, and a cycloalkane are alkane examples of alkanes. Alkane molecules with straight and branched chains have a molecular formula of CnH2n+2, and those with cyclic chains are CnH2n.
Related Topics link
Groups and Alkyls
A carbon-hydrogen bond in an alkane molecule becomes a carbon-substituent bond when a substituent like halogen bonds to it. The process of methane reacting with chlorine forms chloromethane, which is a new compound. CH3 atoms are joined to chlorine atoms in the new compound.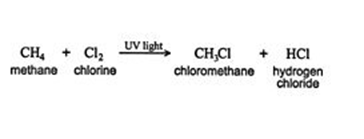
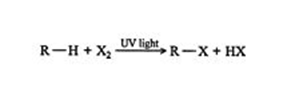
The term alkyl group refers to the removal of hydrogen from one bond in a diketone. This Alkyl group is also commonly represented by the letter R, as opposed to the letter X representing halogens. This reaction between methane and chlorine can be generalized as
Also check-
Frequently Asked Questions (FAQs)
The first four alkanes are methane (CH4),ethane(C2H6) propane(C3H8), butane (C4H10).
Alkanes consist of hydrocarbon atoms with only one bond. Alkanes are divided into three types: linear straight alkanes, branched alkanes and cyclic alkanes.
A neutral alkane usually does not have a functional group but is composed of a component without one. Alkenes are functional groups composed of carbon-carbon double bonds.
Alkanes are a class of hydrocarbons that consist solely of carbon (C) and hydrogen (H) atoms, connected exclusively by single covalent bonds (sigma bonds). They follow the general formula CₙH₂ₙ₊₂, where n is the number of carbon atoms.
Tricarbonyl hydrocarbons are known as alkynes. Their geometric and optical isomerisms are not visible. It is the easiest alkyne to produce, often called acetylene (HC≡CH or C2H2) shown to the right.
Also Read
11 Mar'25 06:34 PM
11 Mar'25 06:27 PM
11 Mar'25 06:25 PM
11 Mar'25 05:54 PM
11 Mar'25 05:27 PM
05 Mar'25 05:23 PM
05 Mar'25 05:18 PM
05 Mar'25 04:59 PM
19 Feb'25 07:36 PM
19 Feb'25 07:33 PM
Articles
Questions related to
Correct Answer: Only I
Solution : The correct answer is Only I
Alkenes are hydrocarbons with at least one carbon-carbon double bond, whether they are branched or unbranched. There is at least one carbon-to-carbon double bond in every alkene. CnH2n is the general formula for alkenes. A family of compounds known as cycloalkanes is made up of carbon atoms joined in a circle. CnH2n+2 is the general formula for cycloalkanes.
Correct Answer: Markovnikov’s rule
Solution : The correct answer is Markovnikov’s rule.
Certain chemical addition reactions can be described by Markovnikov's rule, commonly referred to as Markovnikov's rule. As per this rule, the carbon (C) atom with fewer hydrogen atoms is bound by the nucleophilic X-, whereas the carbon atom with more hydrogen atoms attached to it is bound by the proton.