Aluminium Sulfate - Formula, Preparation, N-factor, Uses, FAQs
Aluminum Sulfate is a chemical compound that is soluble in water. It is predominantly used as a coagulating agent in the purification of drinking water and wastewater treatment plants. It is likewise utilized in the paper manufacturing process. The anhydrous structure of aluminium sulfate is obtained from the volcanic environment and in coal mining. It is obtained in the form of the rare mineral millosevichite.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Aluminium Sulfate formula:
- Preparation:-
- N factor of Al2(SO4)3 and equivalent weight-
- Aluminium Sulfate uses:

In this article, we cover the concept of Aluminium Sulfate which falls under the chapter P-block in Group 13 which is important for boards and JEE Mains Exam and NEET entrance exam .
Aluminium Sulfate is odourless and has a sweet taste. It is a water-soluble chemical compound but is insoluble in ethanol. Aluminium Sulfate emits lethal fumes of sulfur oxide after undergoing the decomposition process.

Aluminium Sulfate formula:
The chemical formula for aluminium sulfate can be represented as - Al2(SO4)3
Dialuminium Trisulfate or Filter alum are some of the other terms used to address aluminium sulphate.
The molar mass of Aluminium Sulfate is 342.15 g/cm3.
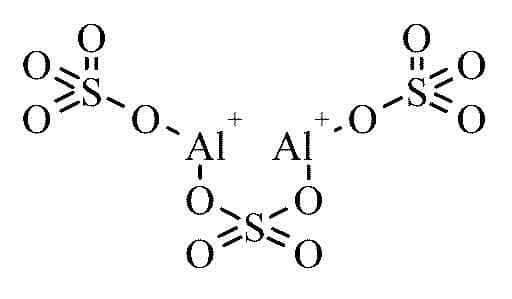
This is the aluminium sulfate formula.
Preparation:-
Aluminium Sulfate can be prepared in the laboratory by the following method-
Aluminium Sulfate is prepared by the addition of aluminium hydroxide-Al(OH)3 to sulphuric acid- H2SO4.
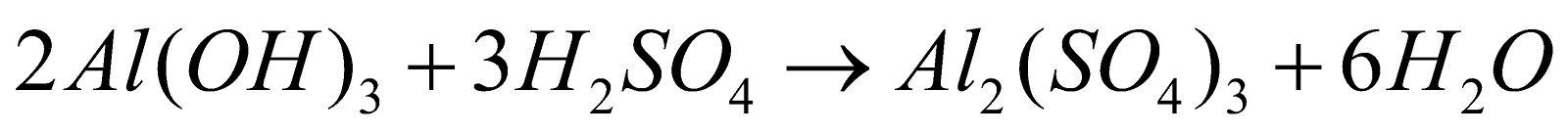
It can also be prepared by heating aluminium in a solution of sulfuric acid.
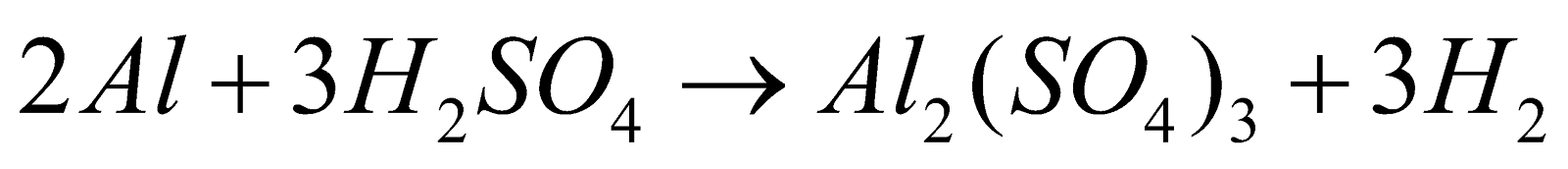
Similarly,Al2(SO4)3 is also manufactured from alum schists, clays or bauxite and cryolite respectively.
Alum schists comprise iron pyrite, aluminium silicate, and several bituminous elements. They undergo the roasting process, where sulphuric acid is formed and made to act on the clay to form aluminium sulphate. A comparable requirement of conditions is produced during the weathering. process; being another process followed for the production of aluminium sulphate.
The mass retrieved is now systematically removed with water and aluminium sulfate solution is prepared of required specific gravity. This solution is made to stand for some duration of time to separate. Calcium and basic iron(III) sulfate are formed. It is then evaporated until iron (II) sulfate crystallises out on chilling. It is then drawn off and evaporated until it gains the required specific gravity and is allowed to stand for some time followed by decantation.
The material is calcinated lightly and then blended with sulphuric acid and water and heated progressively to boiling. If concentrated acid is utilized then no external warmth or heat is required as the formation of aluminium sulfate is an exothermic reaction. The clear solution is extracted after being allowed to stand for a specific extent of time. In this way, aluminium sulfate is extracted from clay or bauxite.
Calcium carbonate is blended with cryolite ore and heated resulting in the formation of sodium aluminate. It is then again heated and precipitated by using sodium bicarbonate or by passing a current of carbon dioxide via the solution. The precipitate is then dissolved in a sulphuric acid solution. In this way, aluminium sulfate is manufactured using cryolite ore.
| Related topics link, |
The cation and the anion in the aluminium sulfate molecule are (Al3+) and (SO3-4) respectively.
The chemical formula of aluminium sulfate can be derived as described below-
The valency of aluminium is 3 and hence it forms a tri cation: (Al3+)
The valency of sulfate ion is 2 and so it forms a
dianion: (SO3-4)
The ions combine in a way to form an electrically neutral salt.
By cross-multiplying their valencies we get –
Al2(SO4)3
The combination of ions takes place in such a way that they form an electrically neutral salt.
N factor of Al2(SO4)3 and equivalent weight-
N factor is the capability of accepting or releasing electrons. This ability is reflected by a change in the oxidation state of the species.
Hence, the n factor for salts can be simply defined as the multiplication of charges of ions present in the salt.
Equivalent weight is defined as the ratio of molecular mass of the charged ions and charges on ionic species (n factor) i.e. ratio of molecular weight of salt to the n factor.
How to find n factor of Al2(SO4)3:
Let us find the n factor of Al2(SO4)3
Al2(SO4)3 compound consists of aluminium and sulfate ions having charges +3 and -2 respectively.
These ions are symbolised as – Al3+ and SO3-4.
By multiplying the charges present in the ions, we get-
3 × 2 = 6
And so, the n factor for Al2(SO4)3 is 6.
Equivalent weight can be given as = M/n factor
= M/6
Related Topics:
Aluminium Sulfate uses:
Aluminum Sulfate functions as a mordant in the dyeing industry. It assists the dye in printing on paper or a piece of fabric.
Aluminium Sulphate's major use in the industrial sector is as a coagulant in water treatment plants. Aluminium Sulfate when mixed with water results in the formation of several forms having varied ionizing levels capable of attracting contaminants present in water and precipitating them out.
Aluminum Sulfate finds its application in paper manufacturing industries as well. It is used to eliminate unwanted foreign particles along with water. It also helps in adhering to materials and neutralizing the charges when used in the pulp itself.
Aluminium Sulfate helps regulate algal growth in water bodies. Aluminium Sulfate being an acidic compound is often used by gardeners to adjust the soil pH.
It is also used as an accelerator and waterproofing agent in the concrete business.
One can find its application as a firefighting foam and fireproofing agent as well.
Also, check-
Frequently Asked Questions (FAQs)
Aluminium Sulphate is an ionic compound composed of Al3+ cation and SO3-4 anion respectively.
Aluminium Sulphate is an eye and skin irritant but not a carcinogen. Aluminium Sulphate when ingested accidentally can be dangerous as it forms hazardous sulphuric acid in the stomach.
The molecular formula for aluminium sulfate is Al2(SO4)3 . It is the symbol of aluminium sulphate.
Aluminium sulphate
342.15 g/mol
Aluminium sulphate is used in paper manufacturing methods, in water purification factories and by gardeners for various reasons.
Aluminium Sulphate is used to eliminate contaminants along with water for the proper manufacturing of paper. It also helps in neutralizing the charges present and can be used in the pulp itself for proper adhering to the material.
Aluminium finds it’s a major application in the water remedy plants where it is used as a coagulant to remove foreign particles to get safe drinking water.
When used as directed, aluminium sulfate is generally considered safe. However, direct exposure in powdered form can cause irritation to the skin, eyes, and respiratory tract. It is important to follow safety guidelines and use protective equipment when handling it.
Aluminium sulfate is used in gardening to acidify soil. It is particularly beneficial for growing acid-loving plants like blueberries and azaleas.
Yes, aluminium sulfate is called as "alum," which is a term that historically referred to a group of related chemical compounds. However, “alum” can also refer specifically to potassium aluminium sulfate (KAl(SO4)2·12H2O), which has different properties and uses.
N factor is the capability of accepting or releasing electrons. A change in the oxidation state of the species reflects this ability.
Also Read
19 Feb'25 11:05 AM
19 Feb'25 11:03 AM
16 Dec'24 11:40 PM
12 Dec'24 05:24 PM
12 Dec'24 12:58 PM
09 Dec'24 11:07 AM
09 Dec'24 11:06 AM
09 Dec'24 10:52 AM
13 Nov'24 07:10 PM

