Xenon Difluoride - Structure, Preparation, Properties, FAQs
In this article we will discuss different xenon compounds, xeof2 structure, xef2 lewis structure, xenon structure.
This Story also Contains
- Xenon Difluoride
- Xenon Structure
- Q Xef2 polar or Nonpolar
- Q Preparation of Xenon Difluoride
- Q Structure of Xenon Difluoride
- Q Xef2 h2o Gives
- Q Xenon Molecular Weight
- Chemical Properties
Xenon Difluoride
Xenon difluoride has the chemical formula XeF2. and The most important property of this compound is that it is a powerful fluorinating agent ( fluorinating agents are the compound that is capable of adding fluorine to any compound ). Xenon difluoride is also one of the stable compounds of xenon. As we can easily observe, xenon fluoride is an inorganic compound from its chemical formula. Xenon difluoride is a moisture-sensitive compound. Due to its sensitivity toward moisture, xenon fluoride will decompose when it comes in contact with water vapour or light. Xenon difluoride is solid with a dense and colourless texture.
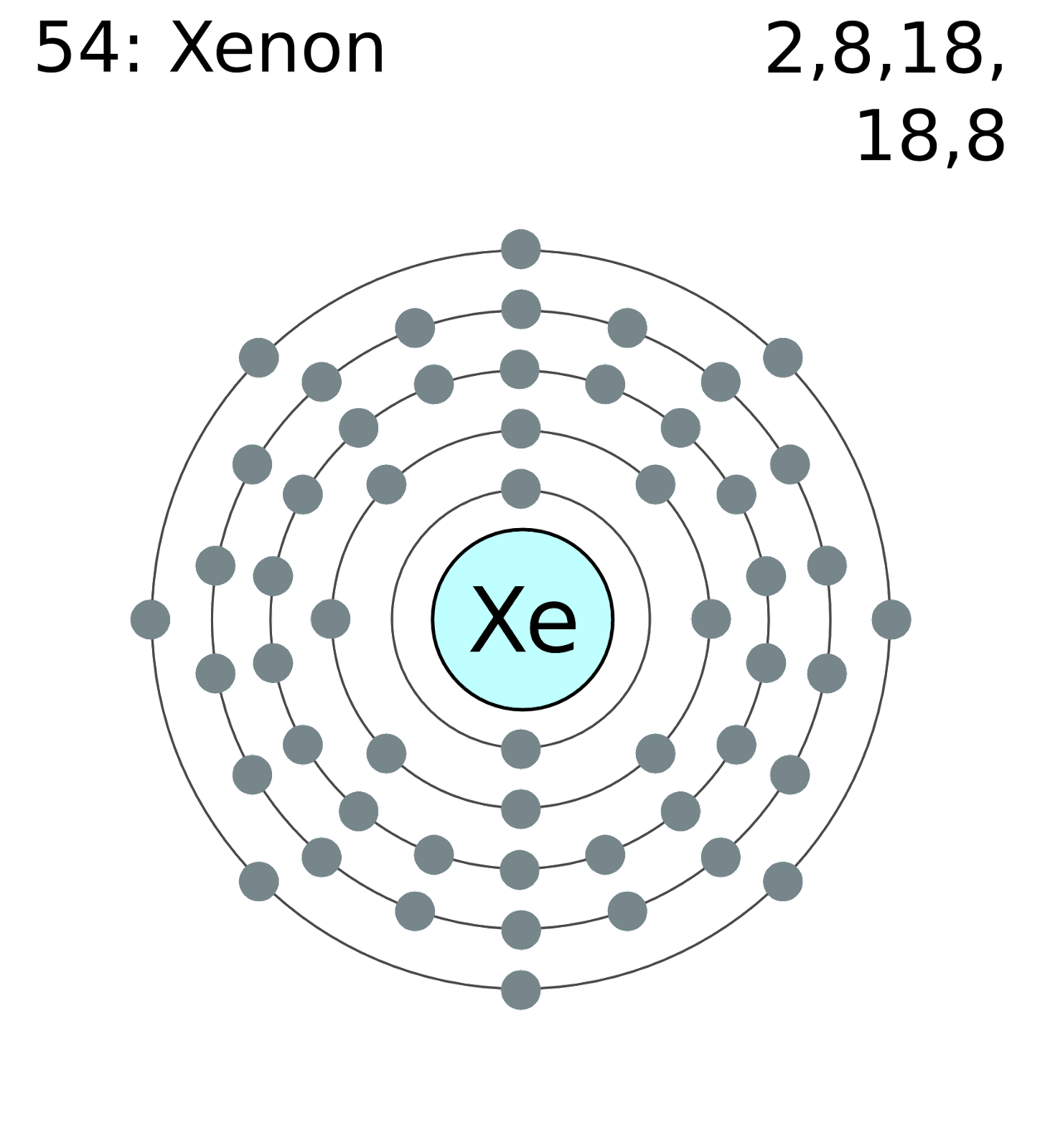
Xenon Structure
XeF2 Lewis Structure
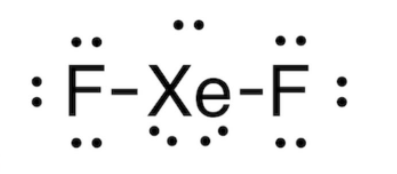
XeOF2 Structure
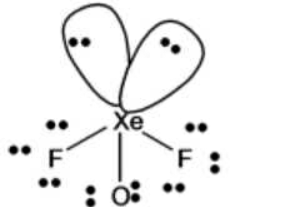
Q Xef2 polar or Nonpolar
The molecule xenon difluoride is nonpolar because of its linear shape geometry and also symmetry is present around I.e. both side of xenon atom
Q. Draw the structure of xef2.
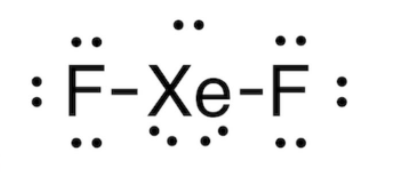
Q Xenon molar mass
Molar mass of xenon is 131.293 u
Q Preparation of Xenon Difluoride
Xe + F2 → XeF2
To proceed this reaction we have to provide energy, it may be in the form of electric discharge, radiation or heat. The product obtained I.e. XeF2 is in solid state. And the product I.e. XeF2 obtained in this reaction is in an impure state, so we use fractional distillation in the vacuum line to get XeF2 in pure form.
It can also be formed from reacting between xenon and dioxygen difluoride
Q Structure of Xenon Difluoride
Xenon difluoride is a linear molecule that has a wavelength of 197 pm in vapour phase and in solid phase xenon difluoride has wavelength of 200pm.
When we observe its solid structure we can easily notice that both the fluorine atom will avoid the equatorial region and also according to VSEPR theory this molecule will have three pairs of unpaired electrons that will be present around the equatorial region of the xenon atom.
Under the pressure of 50 Gpa XeF2 can be converted to XeF4 and we have to note that XeF4 is a semiconductor. The structure of XeF4. will be the same as graphite. And if we further increase pressure I.e. above 90 Gpa then xenon difluoride will change into metallic 3d compound I.e. XeF8
In the XeF molecule Xe—F bonds are weak. There is total bond energy of 267.8 kJ/mol in XeF2 molecule.
Which constitute 184.1 kJ/mol for first bond energy and 83.68 kJ/mol for second bond energy.
One point to note here is that , XeF2. is more stable than KrF2 , Due to its bond energy I.e. only 92.05 kJ/mol.
Q Xef2 h2o Gives
XeF2 is insoluble in water I.e.polar protic solvent. It is soluble in solvents like anhydrous hydrogen fluoride, and acetonitrile, BrF5, BrF3, IF5 without oxidation or reduction. In hydrogen fluoride solubility of xenon difluoride XeF2 is 167 g per 100 g HF.
2XeF2(s)+2H2O(l)→2Xe(g)+4HF(aq)+O2(g).
Q Xef2 Structure
Xenon difluoride is a linear molecule that has a wavelength of 197 pm in vapour phase and in solid phase xenon difluoride has wavelength of 200pm.When we observe its solid structure we can easily notice that both the fluorine atom will avoid the equatorial region and also according to VSEPR theory this molecule will have three pairs of unpaired electrons that will be present around the equatorial region of the xenon atom.
Q Xenon Molecular Weight
The molar mass or molecular weight of xenon atom ( xenon is an ideal gas ) is 131.293 u
Q Synthesis of Xenon Difluoride
Xe + F2 → XeF2
To proceed this reaction we have to provide energy, it may be in the form of electric discharge, radiation or heat. The product obtained I.e. XeF2 is in solid state. And the product I.e. XeF2 obtained in this reaction is in an impure state, so we use fractional distillation in the vacuum line to get XeF2 in pure form.
XeF2 was discovered in 1962 by Chernick. First time it was formed by reaction between xenon and fluorine gas mixture in the electric discharge tube.
It can also be formed from reacting between xenon and dioxygen difluoride.
Q XeF
In the XeF molecule Xe—F bonds are weak. There is total bond energy of 267.8 kJ/mol in XeF2 molecule.
Q Oxidation state of xe in xeof4
Oxidation state of xe I.e. xenon in xeof4 I.e. Xenon oxytetrafluoride is +6 due to higher electronegativity of fluorine.
Xenon difluoride is a linear molecule that has a wavelength of 197 pm in vapour phase and in solid phase xenon difluoride has wavelength of 200pm.
When we observe its solid structure we can easily notice that both the fluorine atom will avoid the equatorial region and also according to VSEPR theory this molecule will have three pairs of unpaired electrons that will be present around the equatorial region of xenon atom.
Q Oxidation number of xenon in xef2
Oxidation state of xenon in XeF2 is +2
| Related topics link, |
Q Solubility of XeF2
XeF2 is insoluble in water I.e.polar protic solvent. It is soluble in solvents like anhydrous hydrogen fluoride, and acetonitrile, BrF5, BrF3, IF5 without oxidation or reduction. In hydrogen fluoride solubility of xenon difluoride XeF2 is 167 g per 100 g HF.
There are many products which can be derived from xenon difluoride XeF2
We can get XeF+ cation by reaction between xenon difluoride with strong fluorine acceptor it can be antimony pentafluoride (SbF5)
XeF2 + SbF5 → XeF+ + SbF−6
If we add xenon gas in the above solution formed then the colour of this solution will be changed from yellow to green and this will happens due to formation of paramagnetic Xe+2 ion.
3Xe(g) + Xe F+(apf) + Sb F5(l) ⇌ 2 Xe+2(apf) + Sb F−6(apf)
And the above reaction is reversible which means that if we remove xenon gas from the solution then xenon caution Xe+2 will revert back to form xenon gas and XeF+. And the colour of the solution will also revert from green to pale yellow.
We can also precipitate dark green crystals from green solution with the help of liquid HF.
Xe+2 (apf) + 4SbF−6 (apf) → Xe+2Sb4F−21 (s) + 3 F− (apf)
Here the bond length between Xe—Xe is 309 pm and as we know that bond length is inversely proportional to bond strength, here in the case of Xe—Xe bond length is big I.e. 309 Pm and that’s why there is a weak bond between Xe—Xe.
Bonding in XeF2. can be described by 3 centred and 4 electron bonds.
Another important use of XeF2 is that it can be used as a ligand in HF solution.
My (AsF6)2 + 4XeF2 → [Mg(XeF2)4] (AsF6)2
In the above complex, six fluorine atoms are coordinated with magnesium atoms. And another four fluorine atom are attribute to the four xenon difluoride ligand and the other two are as a pair of cis-AsF−6 ligands.
Also Read:
Chemical Properties
Xenon difluoride gives substitution reaction when it is reacted with strong protonic acid like perchloric
acid.
The reaction in presence of protonic acid is given below.
![]()
![]()
It will react with NO to give nitrosyl fluoride.![]()
It will react with sulphur trioxide.![]()
Hydrogen will reduce XeF2 to Xe.![]()
With fluorine, it will give higher fluoride.![]()
It will undergo slow hydrolysis when dissolving in water.![]()
It will reacts with SbF5 and AsF5 to form coordination complexes in which XeF2 acts as a donor of F −.![]()
It will fluorinate ethylene to give 1,1–difluoroethane.![]()
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: