Amido And Amide: Structure, Preparation, Classification, Different Types
Amide is classified as any member that belongs to two classes of nitrogen campaigning compound either it can be ammonia or that can be amine. Amide that are formed by the sharing of the electrons are neutral and sometimes they are very weakly acidic substance that are formed by the replacement of hydroxyl group (-OH) of an acid with the help of the amino group (NR2) in which R represents the hydrogen atom or any other of the alkyl groups such as the methyl group or any other alkyl group).
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Structure of Amide:
- Difference Between Amide And Amido Group
- Preparation Of Amides :
- Classification Of Amides:
- Different Types Of Amide And Their Properties:
When the amides formed by the sharing of electrons are derived from the ammonia then they are solid except for the formamide which is a liquid kind amide. Amides that are derived from ammonia and those that contain less than 5 carbon numbers are usually soluble in water. These amides formed by the sharing of electrons pose no free electrons so they are the non conductor of electricity. These have usually high boiling points.
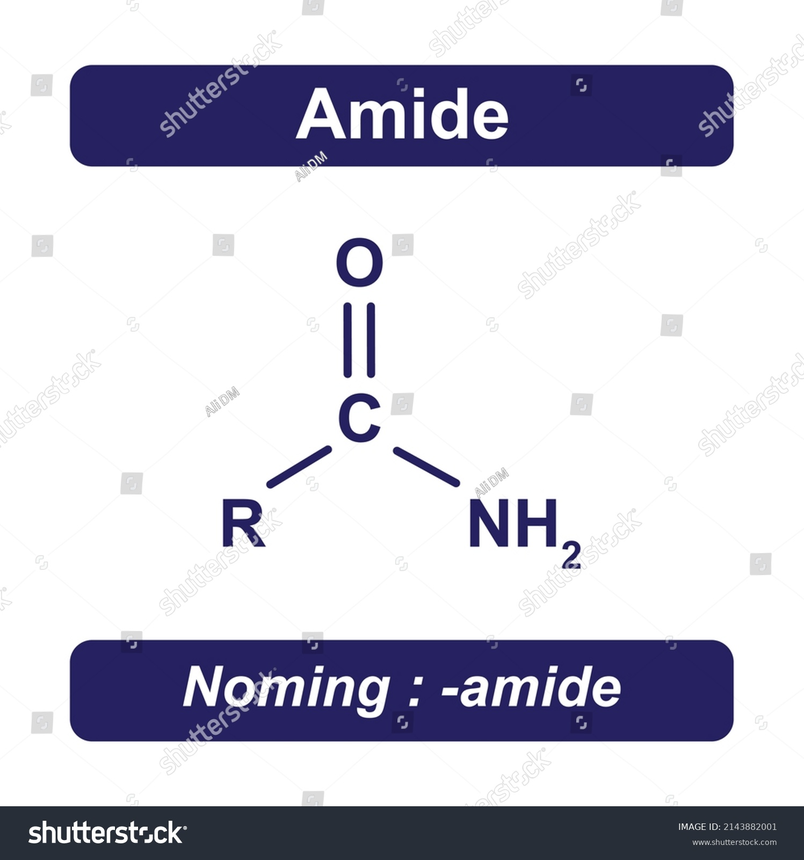
Structure of Amide:
It is defined as a compound that poses a carbonyl functional group and this carbonate functional group is linked to both the amine and hydrocarbon group of the chain. Carbonyl functional group is made up of the double bonded carbon atom and oxygen atom remains attached to that of the carbon atom.
The general structure of amide is as :
R(C=O)NR'R"
In this structure R, R' & R" represents hydrocarbons that are made up of carbon and use hydrogen as substituent.
Difference Between Amide And Amido Group
There is not much difference between these two of the group The only difference that indicates is amido group indicates the presence of an amide group in it.
Preparation Of Amides :
1. Amine acylation :
Generally amides are formed by the reaction of an amine with the help of a carboxylic acid derivative.
In the structure of amine nitrogen possesses a lone pair of electrons and it attacks the electron deficient carbonyl carbon. Now this carbonyl carbon forms the bond with that of the nitrogen atom.
When a bond is formed the elimination of protons from the amine occur and the leaving group forms the amide.
2. Nitrile hydrolysis:
As the name suggests when the nitrile group is lysed with the help of water it is termed as the nitrile(RCN) hydrolysis.
In this reaction the amide group is formed by the partial hydrolysis of the nitriles but some basic or acidic condition should be given.
3. Beckmann rearrangement :
It is the most used amide synthesis method and the most important method.In this reaction amide is formed by the help of the oxime group. The oximes are rearranged to form the amides in the presence of many activating agents such as sulfuric acid or phosphorus chloride.
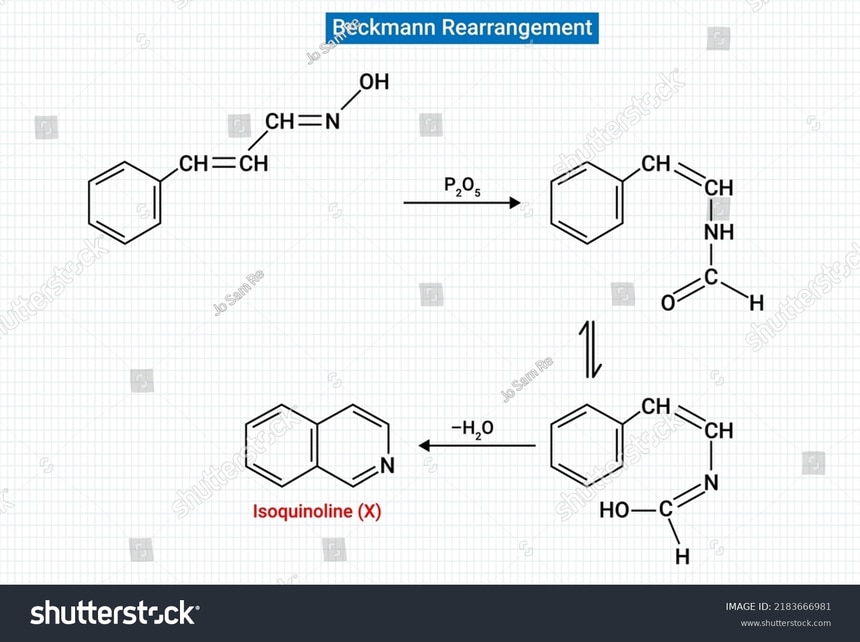
IMAGE URL : https://www.shutterstock.com/image-vector/chemical-structure-beckmann-rearrangement-2183666981
Classification Of Amides:
Amides are generally categorized into three different categories that are :
Primary amide :
These are defined as those amides in which the nitrogen atom substitute of the amide possesses only one hydrogen atom.
Secondary amide:
Those amides in which an amide nitrogen atom is linked to the hydrocarbon substituent of the atom.
Tertiary amide:
These are defined as those amides in which the nitrogen amide is attached to the three carbon.
Cyclic amides:
Cyclic amides are also known as lactam.
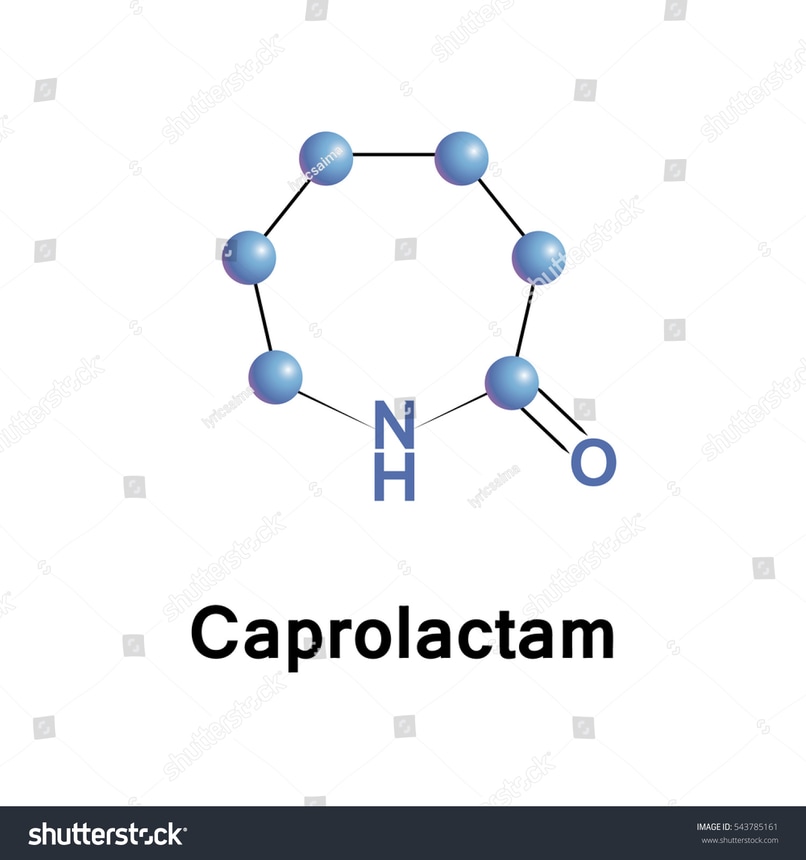
IMAGE URL : https://www.shutterstock.com/image-vector/caprolactam-cpl-organic-compound-colourless-solid-543785161
Different Types Of Amide And Their Properties:
Examples of amides
Formamide
Condensed structure : HCONH2
Melting point : 2°C
Boiling point : 193°C
Solubility in water : Yes formamide is soluble.
Acetamide
Condensed structure : CH3CONH2
Melting point : 82°C
Boiling point : 222°C
Solubility in water : Yes acetamide is soluble.
Propionamide
Condensed structure : CH3CH2CONH2
Melting point : 81°C
Boiling point : 203°C
Solubility in water : Yes it is soluble
Benzamide
Condensed structure : C6H5CONH2
Melting point : 132°C
Boiling point : 290°C
Solubility in water : soluble only in some amount
Basicity of amides:
When compared with the amines, amides are found to be a very weak basic structure.Conjugate acid of amines possesses the pKa of roughly 9.5, and conjugate acid of an amide has a pKa of around 0.5.
So we can observe amides do not possess clear acid–base characteristics in water. The carbonyl carbon is an electron withdrawing group so it withdraws electrons from the amide and so explains the lack of basicity. But when compared with other groups such as carboxylic acids, esters, aldehydes, and ketones (their conjugate acids' pKas range from 6 to 10) aminde are found to be more basic than these groups.
Frequently Asked Questions (FAQs)
When the amides formed by the sharing of electrons are derived from the ammonia then they are solid except for the formamide which is a liquid kind amide. Amides that are derived from ammonia and those that contain less than 5 carbon numbers are usually soluble in water.
When many amide groups are joined together by the help of covalent bonds these are termed as polyamides. They are present in a protein rich diet.
Hybridization is defined as the intermixing of atomic orbitals to form a hybrid orbital. Hybridization of amides is found out to be sp3. We can explain it as the electrons of three p orbitals are engaged on three atoms: oxygen, carbon and nitrogen and s orbital of hydrogen are on the same plane and delocalized.
Lower amides that are amides with lower number of carbon atoms are highly soluble in water as they are involved in hydrogen bonding with that of water. They act both as electron donor and electron acceptor as they have presence of both Nitrogen and oxygen.
Lactam amides are known as cyclic amides.
Also Read
27 Sep'24 05:44 PM
27 Jan'24 11:41 PM
27 Jan'24 11:57 AM
26 Jan'24 10:37 PM
24 Nov'22 06:21 PM
18 Jul'22 03:38 PM
18 Jul'22 03:35 PM