Amines - Notes, Topics, Formula, Books, FAQs
Organic compounds containing nitrogen or amines are an important class of organic compounds formed by replacing one or more hydrogen atoms of ammonia with an alkyl or aryl group. They exist in nature in various forms like proteins, vitamins, hormones, etc. In the form of amino acids, these amines are very important for our body. According to the IUPAC system, the naming of the amines is done by first naming the alkyl group and then adding amine in the end, for example, methylamine. Aromatic amines are named as the derivative of the simplest aromatic amine i.e., aniline, for example, 2-Bromoaniline.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Important Topics - Organic compounds Containing Nitrogen
- Overview Of The Chapter
- Classification Of Amines
- Preparation Of Amines:
- Physical Properties
- Chemical Reactions
- How To Prepare For Amines?
- Prescribed Books
In this chapter, you will study the structures of different types of amines, their preparation, physical properties, chemical reactions, etc. In real life, we see compounds of amines in bulk form but we hardly become inquisitive in their formation and chemical properties. This chapter explains all those questions.
This article will give you all the information regarding the amines chapter. This will help you to make your strategies in your preparation and it will also guide you in how to prepare for this chapter and the best-prescribed books.
Important Topics - Organic compounds Containing Nitrogen
Preparation of Amines:
Amines are the class of organic compounds produced from ammonia NH₃ by replacement of one or more hydrogen atoms with either alkyl or aryl groups. They are classified into three categories: primary, secondary, and tertiary amines. There are various preparations of amines such as Curtuis reaction, Schmidt reaction, loosen reaction, and many more.
Preparation And Properties Of Aromatic Nitrocompounds:
Nitro compounds are highly polar and hence soluble in polar solvents. They usually have higher boiling points than the non-nitro derivatives since there are strong intermolecular forces. Preparation of aromatic nitro compounds is the process of the nitration of aromatic hydrocarbons. This is usually an electrophilic substitution where an aromatic compound reacts with a nitrating agent; commonly, this is a mixture of concentrated nitric acid and sulfuric acid.
Test For Amines:
There are various tests for amines such as when any primary amine(aliphatic or aromatic) is heated with chloroform and alcoholic potassium hydroxide solution, isocyanide(carbylamine) is formed which has a very unpleasant smell. This test is called the carbylamine test or isocyanide test.
Basicity Of Amines:
Basicity is defined as either the acceptance of protons or the donation of electron pairs by a substance. Amines are classified according to the number of groups attached to the nitrogen atom: primary, secondary, and tertiary amines. And the basic nature is due to the presence of an unshared pair of electrons on a nitrogen atom. This lone pair of electrons is available for the formation of a new bond with a proton or Lewis acids
Overview Of The Chapter
In this chapter, there are various important topics that you must understand completely:
Structure Of Amines:
In amines, the nitrogen is in sp3 hybridisation with 3 sigma bonds and 1 lone pair of electrons. Amines possess the tetrahedral geometry but the bond angle in its structure is always less than 109.50 because nitrogen atom has a lone pair of electrons which reduces its bond angle.
.png)
Classification Of Amines
Amines can be classified into three categories as follows, depending on the number of alkyl or aryl groups attached to the nitrogen atom:
.png)
Preparation Of Amines:
Amines can be prepared from the following methods:
- Reduction Of Nitro Compounds: In this reaction, the nitro compounds are reduced by the (H2 / Pd) reagent to amines.
.png)
- Reduction of Nitriles: In this reaction, nitriles are reduced to amines by another equally strong reducing agent i.e (H2 / Ni).
.png)
- Hoffmann Bromamide Degradation Reaction: In this reaction, the amide is treated with bromine in the presence of an aqueous solution of NaOH. This reaction converts amide to a primary amine with 1 carbon less than the amide.
Physical Properties
- Lower aliphatic amines are gases with a fishy smell and higher aliphatic amines are liquid.
- Lower aliphatic amines are soluble in water because of the capability of hydrogen bonding with the water molecules.
- Amines are soluble in organic solvents as well such as alcohol, ether, benzene, etc.
- The boiling point of amines follows the order below:
Primary > Secondary> Tertiary - Intermolecular interaction of amines is more prevalent in primary amines than in secondary amines and this interaction is absent in tertiary amines.
Chemical Reactions
- Alkylation Of Amines: In this reaction, amines are reacted with alkyl halides as shown below in fig. The product formed in this reaction is 10 higher amine.
.png)
- Acylation Of Amines: In this reaction, anhydrides are reacted with amines and the product formed in this reaction is amide.
.png)
- Carbylamine Reaction: In this reaction, primary amines are reacted with chloroform in the presence of potassium hydroxide and the product formed is isocyanides as shown in the figure given below.
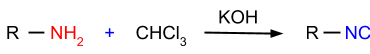
- Reaction with Nitrous Acid: In this reaction, amines are reacted with nitrous acid and form diazonium salt or alcohol, depending on which type of amine is reacting with nitrous acid.
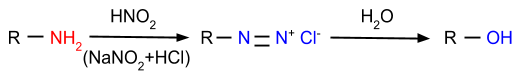
Applications
In the real world, amines are largely used for many applications as follows:
- Methylamines are used for making various agricultural products such as herbicides, insecticides, miticides, etc.
- There are various kinds of amines such as MEA, DEA, DGA, and others are used in industries for removing carbon dioxide and hydrogen sulfide.
- Aromatic amines are used for the production of dyes.
- Amines are used in various kinds of drugs such as chlorpheniramine, ephedrine, amitriptyline, etc.
How To Prepare For Amines?
This chapter is part of organic chemistry. It is completely theory-based. You are not supposed to memorize any formulas and numerical practice to get a good hold of this chapter. All you just need to remember is reaction mechanisms and important reactions.
For this chapter, first, you must have your basic concepts completely clear that you can learn from the chapter- "Some basic principles of Organic Chemistry". For this, you must go through Unit 12 of the NCERT book 11th class part II thoroughly.
- In a nutshell, it can be said that although this chapter is lengthier than other chapters, it is a very simple and straightforward one. So always say a "Big YES" to this chapter.
Prescribed Books
For this chapter, first, the NCERT book is best for initial-level preparation as well as for board exams. Now, after this, if you want to prepare for competitive exams like JEE and NEET, then these are the best books for you - Morrison and Boyd and R.K Gupta by Arihant publication. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Related Topics,
- Ammonia
- Hinsberg Reagent and Test
- Carbylamine Reaction Mechanism
- NH4OH Ammonium Hydroxide
- 2-4 Dinitrophenylhydrazine
- Zwitterion
- Ammonium Phosphate
- Anilines
- Preparation of Ferrous Ammonium Sulphate
Frequently Asked Questions (FAQs)
Nitrogen-containing compounds are chemical compounds that comprise nitrogen atoms bonded to other elements, such as carbon, hydrogen, oxygen, and
The most common types include:
- Amines: Compounds derived from ammonia with one or more hydrogen atoms replaced by hydrocarbon groups.
- Amino acids: Organic compounds that combine to form proteins, containing both an amino group (–NH2) and a carboxyl group (–COOH).
- Nitriles: Compounds containing a cyano group (–C≡N).
- Nitrosamines: Compounds formed by the reaction of nitrites with amines, some of which can be carcinogenic.
- Nitrates and nitrites: Inorganic compounds that contain the nitrate (NO3−) or nitrite (NO2−) ion.
The solubility of amines in water varies based on their structure. Generally, lower molecular weight amines (especially primary and secondary) are soluble in water due to their ability to form hydrogen bonds. As the hydrophobic carbon chain length increases, solubility tends to decrease.
The key difference is that amines are derivatives of ammonia with an attached alkyl or aryl group, whereas amides are formed by the reaction of carboxylic acids with amines, where the nitrogen atom is bonded to a carbonyl group (C=O). Amides generally have different properties and reactivity than amines.
Yes, amines can participate in hydrogen bonding due to the presence of the nitrogen atom, which has a lone pair of electrons. This ability can significantly affect the physical properties of amines, such as their boiling points and solubility in water.
Also Read
20 Dec'24 05:34 PM
27 Sep'24 05:44 PM
27 Jan'24 11:41 PM
27 Jan'24 11:57 AM
26 Jan'24 10:37 PM
24 Nov'22 06:21 PM
18 Jul'22 03:38 PM
Articles
Questions related to
Correct Answer: Phthalaldehyde
Solution : The correct answer is Phthalaldehyde.
Phthalaldehyde is a dialdehyde in which two formal groups are attached to adjacent carbon centres on a benzene ring. It forms a fluorescent conjugation product with primary amines. It is used as a disinfectant, mainly for dental and medical equipment.