Amino Acids: Definition, Benefits, and Food Sources
Amino acids are the very building blocks of proteins, and proteins are the most fundamental macromolecules in any living organism. These organic compounds perform critical functions not only towards the structure of cells but also in various physiological activities, as diverse as muscle contraction and the activation of the immune system. Imagine a world without amino acids: no enzymes catalyzing the biochemical reactions, no antibodies to fight off disease-causing invaders, and no hormones regulating body functions.
This Story also Contains
- Understanding Amino Acids
- Amino Acid Types
- Test for amino acids and proteins
- Relevance and Applications of Amino Acids
- Some Solved Examples
- Summary

Therefore, this article will provide an inside look at the world of amino acids, exploring definitions, types, and real-life applications. We will start with what an amino acid is and follow this by looking at the importance of amino acids in biological systems. This will be followed by a critical look at different types of amino acids and their roles in protein synthesis. Finally, the practical implications of amino acids in terms of nutrition, medicine, and biotechnology will be explained. By the time you have finished reading this article, you will know about amino acids and their importance in both academics and everyday life.
Understanding Amino Acids
Amino acids are defined as organic molecules composed of carbon, hydrogen, oxygen, and nitrogen. They are the basic units linked together to form proteins, which perform a wide range of biological functions. Each amino acid contains a central atom of carbon bonded to an amino group–NH2, a carboxyl group -COOH, a hydrogen atom, and a side chain, the R group that confers the special characteristics of each amino acid. Now, there are 20 standard amino acids, and they can be divided into two major groups: essential and non-essential amino acids. There are those amino acids, like the essential ones, which cannot be produced in the organism, hence due to be ingested with food, and non-essential ones which are synthesized in the organism. These amino acids in a protein, in terms of their sequence and arrangement, as induced by their chemical properties, determine the structure and functions of the protein. All amino acids are therefore very important for life processes such as metabolic processes, immune response, and cellular repair.
Amino Acid Types
Amino acids can be classified based on the side chains, which influence their behaviour and roles in the body. The main classifications are:
1.Essential Amino Acids -
These are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. They have to be ingested through food like meat, eggs, milk products, and some plant proteins.
Amino acids have amino $-\mathrm{NH}_2$ and carboxy1 $(-\mathrm{COOH}$ functional groups. Based on the relative position of the two functional groups in the alkyl chain, the amino acids are categorised as $\alpha, \beta, \gamma, \delta$ and so on. On the hydrolysis of proteins, only α-amino acids are formed. Amino acids may also contain other functional groups.

Amino acids which can be synthesized in our body are known as non-essential amino acids while those that can not be synthesized in our body are known as essential amino acids.
- They are usually colourless, water-soluble, high melting and crystalline solids.
- Except glycine, all other naturally occurring amino acids are optically active.
- Most naturally occurring amino acids have L-configuration.
2. Non-essential Amino Acids:
It include alanine, asparagine, aspartic acid, and glutamic acid. These amino acids are synthesized by the body, while they can also be ingested from a wide variety of food. Since, the -NH2 group is basic and -COOH group is acidic, in a neutral solution, it exists in an internal ionic form called a zwitter ion where the proton of-COOH group is transferred to the $-\mathrm{NH}_2$group to form inner salt, also known as a dipolar ion.

The zwitter ion is dipolar, charged but overall electrically neutral and contains both a positive and negative charge. Therefore, amino acids are high-melting crystalline solids moderately soluble in water and amphoteric in nature. Depending on the pH of the solution, the amino acid can donate or accept protons. In the acidic medium, the COO- ion acts as the base and accepts a proton to form the cation(II) while in the basic medium, the +NH3 ion loses a proton to form the anion(III). Thus, ${ }^{+} \mathrm{NH}_3$ group acts as the acid while $\mathrm{COO}^{-}$group acts as the base.
When an ionised form of amino acid is placed in an electric field, it will migrate towards the opposite electrodes. Depending on the pH of the medium, the following three things may happen:
- In an acidic solution(low pH), the positive ion moves towards the cathode.
- In the basic solution, the negative ion moves towards the anode.
- The zwitter ion does not move towards any of the electrodes.
Electrophoresis is a method used for the separation and analysis of amino acids. At an isoelectric point, an amino acid has the least solubility in water. This method is based on pH control and electric charge. The amino acids differ in their isoelectric point.
Amino acids Isoelectric point
(i) Neutral pH lies between 5 - 6.3
(ii) Acidic pH lies between 3 - 5.4
(iii) Basic pH lies between 7.6 - 10.8
3. Conditional Amino Acids
These are normally non-essential but sometimes may be essential in conditions of stress or sickness. Examples include arginine, cysteine, glutamine, tyrosine, and glycine.
Amino acids can undergo esterification and acetylation reactions. However, some important points have to be noted which are listed below:
- Esterification of amino acid should be carried out in an acidic medium so as to protonate the amino group which would otherwise interfere with the esterification reaction.
- Acylation should be carried out in a slightly basic medium as the amino acid might get protonated in the acidic medium and will not give the reaction.
- p-Aminobenzoic acid and o-Aminobenzoic acid do not exist in the form of zwitter-ion due to the lesser basic strength of aromatic amines.
- p-Aminobenzenesulphonic acid can exist in the form of zwitter-ion.
4. Branched-Chain Amino Acids (BCAAs):
This subgroup includes leucine, isoleucine, and valine, which are particularly related to the proper development of muscles and their recovery after exercise.
These classifications become useful in the identification of dietary sources that allow for optimal health and performance, particularly among athletes and people with specific health problems.
Test for amino acids and proteins
There are two types of tests, viz:
1. Ninhydrin test:
This test is given by all proteins and amino acids. When a protein is boiled with a dilute solution of ninhydrin, a blue-violet colour is produced.
2. Biuret test:
On adding a dilute solution of copper sulphate to an alkaline solution of protein, a violet colour is developed. This test is due to the presence of the peptide (-CO-NH-) linkage.
Relevance and Applications of Amino Acids
Amino acids have implications for every area from nutrition to medicine and biotechnology. They are important in nutrition for repairing and building muscles, so this is a focal point when it comes to athletic performances among athletes and many people involved in fitness. For example, BCAAs are typically supplemented in consideration of enhancing exercise performance and reductions in muscle soreness. In clinical settings, amino acids are utilized in therapeutic diets where patients are recovering from surgery or trauma since they support tissue repair and immune function.
Amino acids find applications in medical science in the formulation of drugs and the development of nutritional therapies. For example, some amino acids are used in intravenous feeding solutions for patients who have problems with oral ingestion of food. Also, research is going on to test the potential of amino acids in treating different diseases, such as depression and metabolic disorders, because of their ability to control the synthesis of neurotransmitters.
Biotechnology has also felt the benefits of amino acids. This is especially so within the scope of protein engineering and synthetic biology. Scientists deviate the sequence of amino acids to create proteins with new features that are then employed in, for example, pharmaceutical, agriculture, and environmental science applications. As an example, organisms with genetically manipulated characteristics can be engineered to express proteins toward enhancing the resilience or yield of crops.
In summary, amino acids are not only the basis of life but also part of health and fitness enhancements and technological development. Such multifarious functions call for a balanced diet that contains these compounds to ensure not just physical health but also the possibility for scientific advancement.
Recommended topic video on(Amino acids)
Some Solved Examples
Example 1
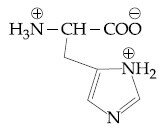
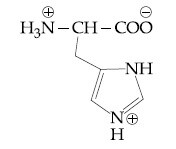
Question: The correct structure of histidine in a strongly acidic solution (pH = 2) is:
1) 
2) 
3) (correct)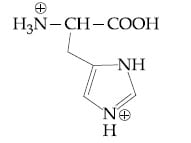
4) 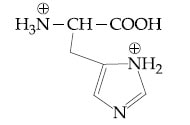
Solution: In a strongly acidic medium (pH=2), all the basic amine groups will be protonated while the carboxylic acid remains in its unionized form. However, one of the nitrogen atoms in the imidazole ring of histidine provides its lone pair for aromaticity and will not be protonated. Therefore, the correct answer is Option (3).
Example 2
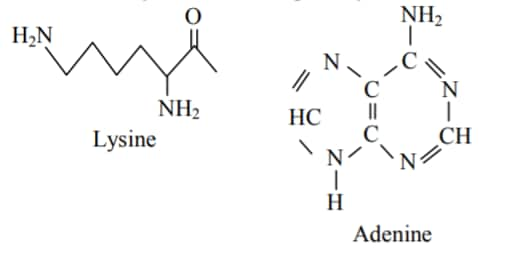
Question: Which of the following will react with $\mathrm{CHCl}_3+$ alc. KOH ?
1)Adenine and Proline
2)Thymine and Proline
3) (correct)Adenine and Lysine
4)Adenine and Thymine
Solution:
$\mathrm{CHCl}_3+$ alc. KOH reacts with those compounds which have -NH2 group.
Only Lysine and Adenine have -NH2 group.
![]()
Therefore, the correct option is (3).
Example 3
Question: Out of the following, which type of interaction is responsible for the stabilization of the α-helix structure of proteins?
1) (correct) Hydrogen bonding
2) Ionic bonding
3) Vander Waals forces
4) Covalent bonding
Solution: Hydrogen bonding is the primary interaction that stabilizes the α-helix structure of proteins. These bonds form between the carbonyl oxygen of one amino acid and the amide hydrogen of another, creating a helical structure. Hence, the correct answer is Option (1).
Example 4
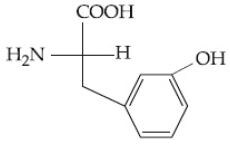
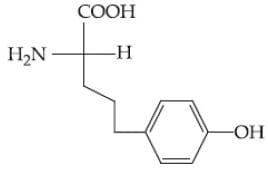
Question: Which of the following is the correct structure of tyrosine?
1) 
2)
3) (correct)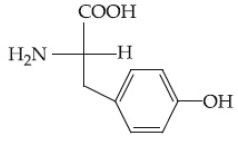
4) 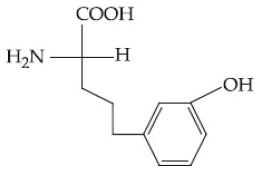
Solution: The structure of the amino acid tyrosine includes a phenolic hydroxyl group attached to the aromatic ring. Therefore, the correct option is (3).
Summary
Amino acids are known as the building blocks of life, functioning essentially as the basic units that comprise proteins. These organic compounds play a crucial role in a host of biological functions, from muscle contraction to immune response. In the current review, the mystifying world of amino acids is viewed in terms of its definition, types, and real-life applications. We started with what an amino acid is and their important role in biological systems, proceeded to types of amino acids and their roles in protein synthesis, and ended up discussing their practical applications in amino acids on nutrition, medicine, and biotechnology.
