Ammonium Chloride - Features, Structure, Types, Formula, Uses, FAQs
NH4Cl is a rare compound with the chemical name Ammonium chloride. It is also known as sal ammoniac, ammonia salt and hydrogen chloride. It is a product of sodium carbonate. In its purest form, it is crystalline salt, white compound. This mixture is very soluble in water and has a mild acidity. It is used in animal medicine to prevent urination in sheep, goats and cattle. When ammonium sulfate and NaCl solutions react together, NH4Cl is produced. When a 5% solution of ammonium chloride is used (by weight) in mixed water, the resulting solution has a pH range of 4.6 to 6.0.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Features of Ammonium Chloride
- Ammonium Chloride Uses
- Health Effects of Ammonium chloride
- Ammonia Preparation- NH3
- Formula of Ammonium Chloride
- Structure of Ammonium Chloride
- Chemical Formula of Ammonium Chloride
- Sublimation of Ammonium Chloride
- Various Types of Bond Available in NH4Cl
Ammonium chloride is an acidizing salt that can be found in the body and urine. The ingredients of ammonium chloride in pH regulation also have a modest effect on abortion. This acid-forming salt also has an expectorant effect on irritating the mucous membranes, making it helpful in coughing.
Features of Ammonium Chloride
Molecular Weight / Molar Mass of ammonium chloride is about 53.491 g / mol
Density is approximately 1.53 g / cm³
Melting point of Ammonium chloride 338 ° C
Related Topics |
Ammonium Chloride Uses
It is used in fertilizers as a source of nitrogen.
It is used medically (especially in cough medicines) as an expectorant.
It is applied to a glue that helps to hold the plank.
It is used in Leclanche cells for powerful solutions.
It is used in food additives - in making bread as an ingredient of yeast.
It is used as an acidifier.
It is used for cooling baths to create low temperatures.
They are used as temporary solutions and ammonia.
It is given to cattle as food additives.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Health Effects of Ammonium chloride
It is not at all dangerous, as overdose is possible. High blood pressure can be caused by ammonium chloride. Anger, shortness of breath, cough, nausea, and headache are all symptoms of ammonium chloride poisoning. Gases have the potential to cause eye discomfort. Chronic exposure can cause chest-like reactions or kidney failure. Ammonium chloride is used as a systemic acidifier in the treatment of severe metabolic alkalosis, in tests of oral acid delivery to identify distal renal tubular acidosis, and in the treatment of several urinary tract urinary tract infections.
Related Topics |
Ammonia Preparation- NH3
Ammonia is easily made in the laboratory by burning salt, ammonium chloride NH4Cl with strong alkali, such as sodium hydroxide or calcium hydroxide.
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3(g)
The gas can also be produced by evaporation of concentrated ammonium hydroxide.
The main commercial method of producing ammonia is the Haber Process, a direct combination of nitrogen and hydrogen under high pressure where there is a catalyst.
Formula of Ammonium Chloride
The ammonium chloride formula is given at the following points. First it is important to remember that ammonium chloride is an inanimate compound. It is a white crystalline salt that dissolves easily in water.
NCERT Chemistry Notes :
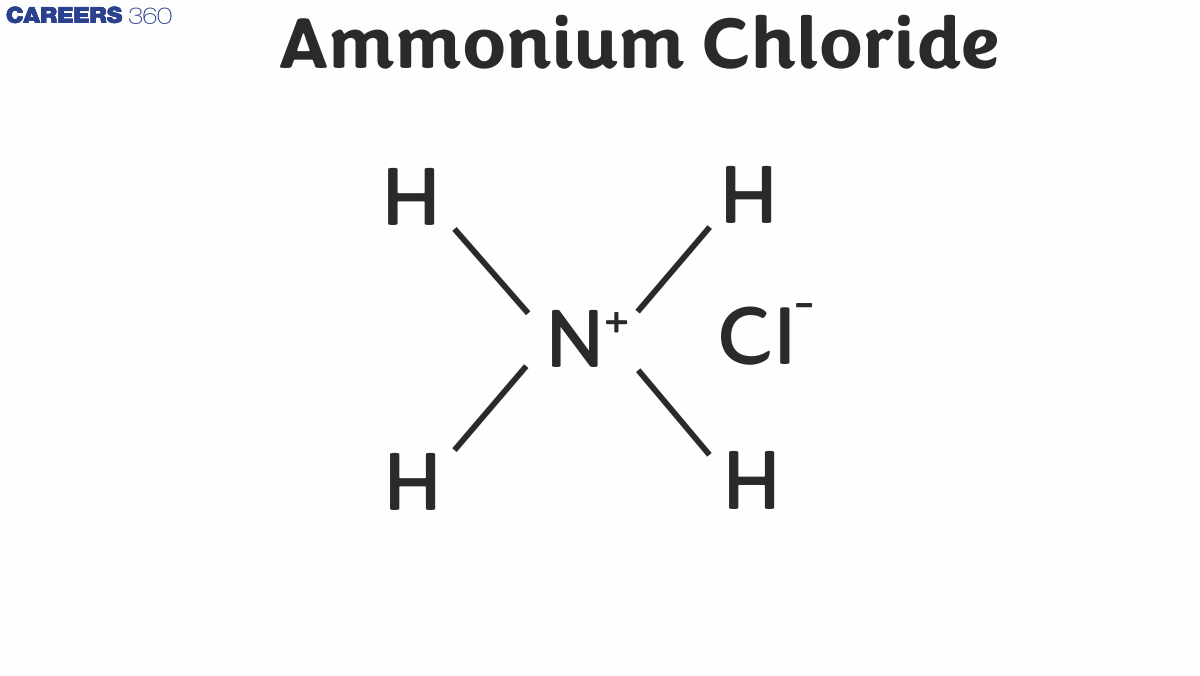
Structure of Ammonium Chloride
Ammonium chloride is a water-soluble and soluble compound. It contains nitrogen ions, four hydrogen atoms, and chloride ions.
Chemical Formula of Ammonium Chloride
The ammonium chloride molecule contains nitrogen, chloride and hydrogen atoms. The chemical or molecular formula of ammonium chloride is as follows-
Ammonium Chloride Chemical Formula = NH4Cl
Ammonium chloride is used in a number of fields such as fertilizers, glue, medicines, food additives, cooling baths, and acidifiers. There are various uses of NH4Cl but their use should be limited due to the environmental risks they cause
What happens to given pure ammonium chloride during the sublimation of ammonium chloride?
Pure ammonium chloride is collected on the inner sides of the funnel in a sublimate form.
Related Topics
Sublimation of Ammonium Chloride
Sublimation is the substance of an object in which it is converted directly from solid to gas or vice versa. Such things are known as sublime.
Take a mixture of ammonium chloride and salt in a Chinese bowl to cover it with a circular turning tube. On the other side of the panel, place a cotton plug to keep smoke out. Now place the china dish on the stove. Since ammonium chloride is low after heating, it will convert directly into steam. This vapor will also turn in the colder part above to form a solid ammonium chloride. In this way, the mixture of ammonium chloride and salt can be separated by sublimation.
When the ammonium chloride is dissolved in water the solution then becomes cold. The change is Endothermic because heat is absorbed.
Various Types of Bond Available in NH4Cl
NH4Cl is a rare compound with the chemical name Ammonium chloride. Common name of ammonium chloride is Sal ammoniac, ammonia salt and hydrogen chloride. Ammonium chloride helps maintain pH and has a mild effect on diarrhea.
In the NH4Cl molecule, an ionic bond is formed between NH4+ and Cl- ions
3 covalent bonds are formed between N and 3H atoms.
A single bond bond is formed between N and one H atom.
Molar mass of ammonium chloride
53.491 g/mol
NH4Cl colour
White Crystalline Solid
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Frequently Asked Questions (FAQs)
Ammonium chloride is used in various industries, including as a fertilizer, in food processing as a food additive (E510), in the manufacture of dry cells (batteries), and as a flux in metalworking and soldering among others.
The formula for ammonium chloride is NH₄Cl.
Ammonium chloride can be harmful if exposed to large quantities or when inhaled, causing irritation to the respiratory tract, skin, and eyes. However, it is generally considered safe in small amounts, especially as a food additive or fertilizer.
The pH of a solution of ammonium chloride (NH₄Cl) ranges between 5.5 to 6.5, making it slightly acidic due to the hydrolysis of the ammonium ion (NH₄⁺) in water.
The famous name for ammonium chloride is sal ammoniac.
Also Read
19 Feb'25 11:05 AM
19 Feb'25 11:03 AM
16 Dec'24 11:40 PM
12 Dec'24 05:24 PM
12 Dec'24 12:58 PM
09 Dec'24 11:07 AM
09 Dec'24 11:06 AM
09 Dec'24 10:52 AM
13 Nov'24 07:10 PM