Ammonium Nitrate: Structure, Preparation, Properties, Uses
Ammonium nitrate is defined as an ionic salt which is made up of the ammonium cation![]() and the nitrate anion
and the nitrate anion ![]() .
.
Chemical Name
The chemical name of Ammonium Nitrate is ![]() .
.
Structure Of Ammonium Nitrate
Ammonium nitrate compounds have many features as an ionic bond between an ammonium ion and a nitrate ion.
The pi electrons in the nitrate ion are delocalized due to resonance and the net charge on this ion is -1 (because the nitrogen atom holds a charge of +1 and each oxygen atom holds a charge of -⅔). Thus, only one ![]() ion can form an ionic bond with one
ion can form an ionic bond with one ![]() ion.
ion.
Chemical Data
Chemical Formula is
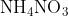
Molar Mass/ Molecular Weight is 80.043 grams per mole
Density is 1.725 grams per cubic centimetre
Melting Point (MP) is 442.8K (169.6oC)
Boiling Point(BP) decomposes at 483K (210oC)
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Chemical Name
- Structure Of Ammonium Nitrate
- Chemical Data
- Preparation Of Ammonium Nitrate
- Ammonium Nitrate Properties
- Uses Of Ammonium Nitrate

Preparation Of Ammonium Nitrate
Ammonium nitrate which is commonly known as ![]() readily dissolves in water by dissociating into its constituent ions. This salt is acidic because it is derived from a weak base that is
readily dissolves in water by dissociating into its constituent ions. This salt is acidic because it is derived from a weak base that is ![]() and a strong acid that is
and a strong acid that is ![]() .
.
Ammonium nitrate ![]() can be prepared from the acid-base reaction between nitric acid and ammonia which is described by the following chemical equation:
can be prepared from the acid-base reaction between nitric acid and ammonia which is described by the following chemical equation:
![]()
The above-given reaction is highly exothermic and proceeds violently. It is also known for its oxidizing powers and is widely used in many explosives used in the mining and construction sectors. NH4NO3 is the key component of ANFO, which is one of the most popular choices for industrial explosives.
Ammonium Nitrate Properties
Physical Properties
Ammonium nitrate is a crystalline solid having a white colour.
Ammonium nitrate has a trigonal crystal structure.
Ammonium nitrate is quite soluble in water and its solubility at 20oC is 150g/100ml. The solubility increases to 1024g/100ml when the temperature is raised to 100 degrees.
The dissolution of Ammonium nitrate (NH4NO3) in H2O is highly endothermic.
Ammonium nitrate has very low shock and friction sensitivities.
Chemical Properties
When Ammonium nitrate (NH4NO3 ) is reacted with the hydroxides of alkali metals then the nitrates of alkali metals are formed along with ammonia.
While heating, this compound decomposes to form nitrous oxide (N2O) and water.
And when exploded, this compound yields N2, O2, and water as the by-products.
Despite being a component of many explosives, ammonium nitrate is not an explosive by itself because it must be mixed with a primary explosive like azide to form an explosive mixture.
Uses Of Ammonium Nitrate
Some applications of this ionic salt are given below.
Ammonium nitrate (NH4NO3) is a key component of many fertilizers because it is quite rich in nitrogen (34%).
This compound does not lose its nitrogen to the atmosphere, as is the case with urea.
And when mixed with fuel oil, it forms an explosive which is known as ANFO. It is one of the most popular industrial explosives.
The dissolution of this compound in water is quite endothermic due to it makes an ideal choice of material in instant cold packs.
Ammonium nitrate is also used in the mining and construction industry as a component of explosives.
Frequently Asked Questions (FAQs)
Ammonium nitrate is not an acid but a salt because the solution is acidic since it is a salt with a weak base (ammonium hydroxide) and a heavy nitric acid.
Ammonium nitrate is sensitive when it is mixed with titanium, tin, or aluminium.
Due to exposure to high concentrations of ammonia in the air, can cause immediate burning of the eyes, nose, throat and respiratory tract that can result in blindness, lung damage or death.
At 400 degrees Fahrenheit temperature ammonium nitrate explodes.
The formula of ammonium nitrate is NH4NO3.
Also Read
09 Dec'24 10:53 AM
30 Sep'24 11:36 AM
30 Sep'24 11:34 AM
30 Sep'24 11:34 AM
30 Sep'24 11:30 AM
30 Sep'24 10:59 AM