Ammonium Oxalate: Introduction, Molecular Weight, Properties, Formula, Uses
In Chemistry, salt is always known by a substance which is made up of the reaction between acid and base. Ammonium oxalate is a colourless salt which is white in colour under the standard conditions and it is considered odourless and non-volatile. Ammonium oxalate is the ammonium salt of oxalic acid which can occur in many plants and vegetables.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Introduction
- Ammonium Oxalate Formula
- Molecular Weight Of Ammonium Oxalate
- Properties of Ammonium Oxalate C_2H_8N_2O_4 .
- Ammonium Oxalate Test
- Uses of Ammonium Oxalate
- Health Hazards Associated with Ammonium Oxalate
Introduction
Ammonium oxalate is mostly used as an analytical reagent and general reducing agent and other oxalates are used as anticoagulants and it is used to preserve blood outside the body. Acid ammonium oxalate can be acidified to pH 3 with oxalic acid which is commonly employed in soil and this chemical analysis to extract iron and aluminium from the poorly-crystalline miners like ferrihydrite, iron (II)-bearing minerals like such as organic matter.
Ammonium Oxalate is known for being an inorganic compound with the chemical. The al formula C2H8N2O4.
Another name for Ammonium Oxalate is diammonium oxalate or diammonium salt.
Ammonium Oxalate is consider as an oxalate salt with ammonium with a strong dicarboxylic acid. Ammonium Oxalate compound consists of ammonium ions and oxalate ions which is in the ratio of 2:1.
Under standard conditions, ammonium Oxalate is colourless to white salt, odourless and non-volatile and also mixes slowly with water. Which can occur naturally in many vegetables and plants.
Ammonium Oxalate Formula
The chemical formula for ammonium oxalate is given by NH_4OOCCOONH_4 ![]() and the molecular formula for Ammonium oxalate is given by C_2H_8N_2O_4
and the molecular formula for Ammonium oxalate is given by C_2H_8N_2O_4 ![]() .
.
The ammonium oxalate name suggests that it will contain only 2 molecules of ammonia and only one molecule of oxalate.
Molecular Weight Of Ammonium Oxalate
The chemical formula of ammonium oxalate is given by C_2H_8N_2O_4 ![]() .
.
So, (12×2) + (1×8) + (14×2) + (16×4) =124.1
Therefore, the molecular weight of ammonium oxalate will be 124.1 g/mol.
Properties of Ammonium Oxalate C_2H_8N_2O_4 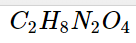 .
.
C_2H_8N_2O_4
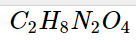 . - Ammonium Oxalate
. - Ammonium OxalateThe Molecular Weight/ Molar Mass of Ammonium Oxalate is 124.1 g/mol
The density of Ammonium Oxalate is 1.5 g/cm3 at 65.3° F
Appearance Ammonium Oxalate is a White solid
The Melting Point (MP) of Ammonium Oxalate is 70°C
Ammonium Oxalate is considered as colourless crystalline solid under the standard conditions with a melting point i.e. M.P of 374.5K in hydrated form and 463K in an anhydrous form and also soluble in water and alcohol but insoluble in ether.
Ammonium Oxalate Test
Oxalic acid can be done by only using a method such as a permanganate titration and atomic absorption methods, etc. As ammonium oxalate mostly helps in preventing and inhibiting the coagulation of blood plasma; so, it can be used in blood tests to prevent blood coagulation and the lead or calcium ions which is present in the blood, as well as other analytes, can be measured by complexing the chemical with specific metals.
Uses of Ammonium Oxalate
Ammonium Oxalate is found in the body of vertebrates and is produced by ascorbic acid or glyoxylic acid metabolism.
Ammonium oxalate can exist as a mineral in the form of Oxammite.
Ammonium oxalate is also used as a reducing agent.
Ammonium oxalate is mostly used as an analytical reagent and to preserve blood outside the body, ammonium oxalate is used as an anticoagulant in these.
Health Hazards Associated with Ammonium Oxalate
Ammonium oxalate dust upon ingestion and excessive inhalation can also cause systemic poisoning which is a hazard to our body.
When it is in contact with the eyes it can irritate.
And contact with the skin can irritate severe burns.
When contact with the skin can cause irritation and most importantly, the physician should be contacted if anyone develops any signs or symptoms in their body and suspects that they are caused by exposure to ammonium oxalate.
Frequently Asked Questions (FAQs)
Yes, ammonium acid is soluble in water because ammonium oxalate ionic compound is usually found as a monohydrate salt that is the reason for solubility in water.
The name of NH_4C_2O_4 ![]() is ammonium oxalate.
is ammonium oxalate.
Yes, ammonium oxalate is an ionic compound because it is an ammonium salt which consists of ammonium and oxalate ions with the ratio of 2:1.
Ammonium oxalate has many uses; it is used in blood tests where it helps to prevent or inhibit the coagulation of blood plasma and this compound can also be complexed with certain metals which quantify the Pd or Ca ions and this ions present in blood and other analytes.
When ammonium oxalate is heated it decomposes on heating and burning which produces toxic and corrosive fumes which are harmful for our body and it includes ammonia and nitrogen oxides also.
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM