Anomalous Behaviour of Boron
Boron is derived from the element found in everyday-use materials: laundry detergents and glass. Boron compounds are contained in the display screen of a smartphone and an interface that treats and makes water safe to drink. These unique properties set it apart from the rest of its group members in the periodic table.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Anomalous Boron
- Boron's Anomalous Behaviour - Different Aspects
- Impact and Applications of Boron's Anomalous Behaviour
- Some Solved Examples
- Summary
Certain important trends can be observed in the chemical behaviour of group 13 elements. The tri-chlorides, bromides and iodides of all these elements being covalent are hydrolysed in water. Species like tetrahedral [M(OH)4]- and octahedral [M(H2O)6]3+, except in boron, exist in an aqueous medium.
The monomeric trihalides, being electron-deficient, are strong Lewis acids. Boron trifluoride easily reacts with Lewis bases such as NH3 to complete octet around boron.
It is due to the absence of d orbitals that the maximum covalence of B is 4. Since the d orbitals are available with Al and other elements, the maximum covalence can be expected beyond 4. Most of the other metal halides (e.g., AlCl3) are dimerised through halogen bridging (e.g., Al2Cl6). The metal species completes its octet by accepting electrons from halogen in these halogen-bridged molecules.
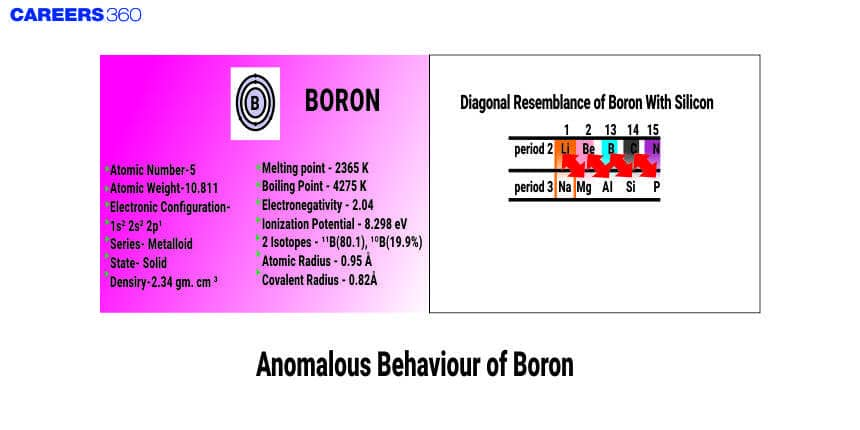
Anomalous Boron
Boron is an element with atomic number 5 and at the metalloids group. It is very hard, having a high melting point. It is one of the Group 13 nonmetals that include aluminium, gallium, indium, and thallium. This could be due to the small atomic size, high ionization energy, and lack of d-orbitals. Small atomic radii result in compact structures of the boron atom with strong covalent bonding and, hence, unique physical and chemical characteristics. For example, it forms covalent bonds and not metallic bonding as in Al and Ga. It may well be that understanding some of these major differences would go a long way in viewing the peculiar behaviour of Boron.
Boron's Anomalous Behaviour - Different Aspects
Many properties of boron differ significantly from the other members of that group. It primarily forms a covalent type of compounds, such as borates and boranes, rather than the ionic type of compounds. On the other hand, in sharp contrast with aluminium oxide, which is ionic, the covalent oxide of boron is stable, B₂O₃, similar to carbon dioxide, CO₂. One more interesting property of the behaviour of boron is that compactness and high electronegativity let it create complex structures, which are in the form of boron clusters and polymers. Besides this, whereas aluminium reacts quickly with acids and bases, so boron is passive towards these—another dislike. These reasons help show the variety and importance of how boron does not follow the trend for a Group 13 compound.
Impact and Applications of Boron's Anomalous Behaviour
The great properties of boron raise important applications across a wide range of fields. Most industrially, its compounds find application in the making of borosilicate glass known for its robustness and ability to withstand drastic thermal shock. Its fibres have a great ratio of strength to weight and are, thus, importantly applied in aerospace engineering. These semiconductors have important applications in making p-type materials in the construction of various types of electronic devices, like transistors and solar cells. The role of boron in medicine is also quite important, for BNCT is an experimental neutron-capture therapy for cancer. At the same time, it is an essential micronutrient required for plant growth in agriculture, for it has a role in cell wall formation and nutrient transport. Its extensive applications justify the value it has for modern technology and science.
Recommended topic video on (Anomalous Behaviour of Boron)
Some Solved Examples
Example 1
Question:
Which one of the following anions cannot be formed by a boron?
- BF63−
- BH4-
- B(OH)4−
- BO2−
Solution:
Boron cannot expand its octet due to the non-availability of d-orbitals. The maximum covalence of Boron cannot exceed 4. Due to the absence of low-lying vacant d orbital in B, sp3d2 hybridization is not possible and hence BF63− will not form.
Hence, the answer is option (1).
Example 2
Question:
The anomalous behaviour of Boron is due to:
- The smallest size in the group
- High ionisation energy
- Does not have vacant d - orbital
- All of the above
Solution:
The anomalous behaviour of boron is due to its smallest size in the group, high ionization energy, and the absence of vacant d-orbitals in its valence shell.
Hence, the answer is option (4).
Example 3
Question:
Which of these statements is not true?
- NO+ is not isoelectronic with O2
- B is always covalent in its compounds
- In an aqueous solution, the Tl+ ion is much more stable than Tl3+
- LiAlH4 is a versatile reducing agent in organic synthesis.
Solution:
Boron is always covalent in its compounds.
Hence, the answer is option (2).
Summary
The large field of applications underlines the goodness of boron for modern technology and science. The anomalous behaviour was explained due to the small atomic size, high ionization energy and, thirdly, the absence of d-orbitals by boric acid. It is due to the formation of stable covalent compounds, resistance to acids and bases, and the nature of complex formation that special characteristics are denoted towards boron. Made by the properties, boron became an undeniable material in various industrial, electronic, medicinal, and agricultural uses. Not only would its importance add to more exploration in the realm of chemistry, but it would also bring new avenues with technological development.
Frequently Asked Questions (FAQs)
Boron is small in atomic size; its ionization energy is high because there are no d-orbitals, hence there will be strong covalent bonding. Moreover, it shows quite several unique chemical and physical properties. All of the above features make the given chemical very different from all the other Group 13 elements.
It is mainly used to make borosilicate glass, which is at once thermally resistant and at the same time very hard. This chemical is also found in various laundry detergents and water purification and it is part of electronic instruments, for example, in transistors, solar cells, etc.
The rigidity ensured by the boron is due to its strong covalent bonding and stable covalent compounds as opposed to aluminium which readily reacts with acids and bases hence relatively less reactive to both acidic and basic conditions.
This becomes one of the very useful microelements for plants. It plays a key role in the plants during cell wall formation, transportation of nutrients, and overall health of the plants. Low levels of boron will lead to retarded growth and reduced crop yields.
Boron is used for experimental therapy for cancer. Boron neutron capture therapy works to pinpoint cancer by delivering boron compounds into the site of a tumour.
Also Read
19 Feb'25 11:05 AM
19 Feb'25 11:03 AM
16 Dec'24 11:40 PM
12 Dec'24 05:24 PM
12 Dec'24 12:58 PM
09 Dec'24 11:07 AM
09 Dec'24 11:06 AM
09 Dec'24 10:52 AM
13 Nov'24 07:10 PM

