Arsenic: Properties, Characteristics, Uses, Effects on Health
The chemical element arsenic has the atomic number 33 and the letter As as its symbol. Numerous minerals include arsenic, which can also be found as a pure elemental crystal. It typically occurs alongside sulphur and metals. Metalloids include arsenic. There are several allotropes of it, but the grey form with a metallic look is the only one that is significant for the industry.
This Story also Contains
- Properties
- Chemical And Physical Characteristics
- Arsenic's Uses
- Effects Of Arsenic On Health
- Arsenic's Effects On The Environment

Arsenic is mostly used in lead alloys (for example, in car batteries and ammunition). A typical n-type dopant in semiconductor electronic devices is arsenic. Additionally, it is a part of the semiconductor III-V compound gallium arsenide. Pesticides, treated wood products, herbicides, and insecticides are all made with arsenic and its byproducts, particularly trioxide.
Properties
The valence of arsenic can be -3, 0, +3, or +5. There are two main allotropes of the elemental solid, while there are more allotropes that have been reported. While grey or metallic arsenic has a specific gravity of 5.73, yellow arsenic has a specific gravity of 1.97. The most common stable form of arsenic is grey, which has a melting point of 817°C (28 atm) and a sublimation point of 613°C. Semi-metallic grey arsenic is an extremely brittle solid. It is crystalline, steel-grey in colour, easily tarnishes in the air, and when heated, it quickly oxidises to arsenic oxide (As2O3) (arsenic oxide exudes the odour of garlic). Poisonous compounds made with arsenic exist.
Chemical And Physical Characteristics
The following is a list of arsenic's physical and chemical characteristics:
Arsenic has an atomic number of 33. In other words, the nucleus of an atom of arsenic contains 33 protons.
Arsenic has an atomic mass of 74.92.
At a temperature of 20 °C, it is discovered to be solid.
It appears to be metallic grey.
816.8 °C is its melting point.
Arsenic has a boiling point of 614 °C.
At 616 °C, it reaches its boiling point.
The rhombohedral crystal structure is seen.
It has a density of 5.72 g/cm3.
Arsenic combines with wet air to produce the arsenic oxide, which coats the element's surface in a dark coating. You should take note of the fact that arsenic does not react with water or dry air in this situation. Below is a list of arsenic reactions with oxygen.
4As_{(s)} + 5O_{2(g)} \longrightarrow As_4O_{10(s)}
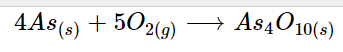
4As_(s) + 3O_{2(g)} \longrightarrow As4O_{6(s)}
![]()
Arsenic interacts with fluorine to produce the colourless compound arsenic pentafluoride in the presence of halogens.
2As_{(s)} + 5F_{2(g)} \longrightarrow 2AsF_{5(g)}
![]()
Under regulated circumstances, arsenic can also react with other halogens including chlorine, bromine, and iodine to produce arsenic trichloride, which is colorless, arsenic tribromide, which is pale yellow, and arsenic triiodide, which is red. The following are responses.
2As_{(s)} + 3C_{l2(g)} \longrightarrow 2AsC_{l3(l)}

2As_{(s)}+ 3Br_{2(g)} \longrightarrow 2AsBr_{3(s)}
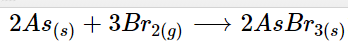
Arsenic's Uses
A tiny quantity of this element is utilized in alloys and most of it is used in compounds.
In the past, it was also used to prepare the lead shot, though the amount used for these purposes has declined. These applications can easily release arsenic into the environment. To create transistors, germanium and silicon are given a certain amount. Arsenic-based gallium arsenide is used to create light-emitting diodes (LEDs). These LEDs create illuminated numbers on clocks, watches, handheld calculators, and other electronic gadgets.
Effects Of Arsenic On Health
One of the most dangerous substances that can be found in arsenic. Inorganic arsenic bonding does exist on earth naturally, despite having a harmful effect. Arsenic exposure in humans can happen through food, drink, or air. Skin contact with arsenic-containing water or soil can also lead to exposure.
Arsenic levels in food are relatively low because it is not added because of their toxicity. However, because fish absorb arsenic from the water they live in, levels of arsenic in fish and seafood may be high. Luckily this is mostly the rather innocuous organic form of arsenic, but fish that have significant amounts of inorganic arsenic may be a hazard to human health.
Arsenic's Effects On The Environment
Due to human intervention in the arsenic cycle, there is now a significant amount of arsenic in the environment as well as in living things. Arsenic is mostly released during the manufacture of copper, although it is also produced during the production of lead and zinc and in agriculture. Once it has gotten into the environment, it cannot be eliminated, so the amounts we contribute can spread and affect people and animals' health in many different parts of the world.
Frequently Asked Questions (FAQs)
For solid-state devices, arsenic is used as a doping agent in semiconductors (gallium arsenide). It can be used for pyrotechnics, hardening shot, and bronze. Speciality glass can be made with arsenic compounds, and it can also preserve the wood.
Arsenic is particularly dangerous when it is in its inorganic form. Contaminated water used for drinking, food preparation, and agriculture irrigation poses the most threat to human health from arsenic. Arsenic poisoning from food and drink can eventually cause cancer and skin blisters.
A toxin that is frequently utilised is arsenic (As). Few individuals are aware that As is frequently utilised in medication. In the past, as well as its components, have been used to treat conditions like diabetes, psoriasis, syphilis, skin ulcers, and joint issues.
Apple, pear, and grape juice may contain tiny amounts of arsenic because the substance is present in the fruit. Juice from concentrate may contain more arsenic if blended with water that contains arsenic.
A dangerous element is arsenic. There is proof that athletes have died from its high dosage in the form of formulations. Carcinogenic inorganic arsenic exists. Arsenic is primarily found in drinking water and agricultural products that we consume. Arsenic poisoning, which can have an impact on a variety of organs and organ systems, is brought on by high levels of arsenic in the body. Skin, the cardiovascular system, the immunological system, the endocrine system, the neurological system, the respiratory system, etc. may all be impacted. Organic arsenic compounds are less toxic than elements of arsenic and inorganic arsenic compounds.
Questions related to
On Question asked by student community
Correct Answer: Overexploitation of ground water in the affected areas
Solution : The correct answer is Overexploitation of ground water in the affected areas.
Arsenic, a naturally occurring element present in the Earth's crust, is ubiquitously distributed in the environment, encompassing the atmosphere, bodies of water, and terrestrial regions. In its inorganic state, it poses severe toxicity risks. Arsenic is naturally present in deep underground water reservoirs, and when there is extensive extraction of groundwater, it can lead to the release of arsenic into the surrounding environment. Additionally, sources of arsenic pollution include high-temperature industrial processes such as those seen in coal-fired power plants, volcanic activity, wildfires, and similar phenomena.
Correct Answer: Extrinsic semiconducter
Solution : The correct option is Extrinsic semiconductor.
When arsenic atoms are introduced into the germanium lattice, it transforms into an extrinsic semiconductor. Arsenic is a pentavalent impurity, with five valence electrons. When an arsenic atom is introduced into a germanium lattice, it forms four covalent connections with the germanium atoms while retaining one additional electron. This electron is weakly bonded to the arsenic atom and is quickly liberated by heat energy, resulting in a free electron. Because of the presence of free electrons in the germanium lattice, it is a better electrical conductor than pure germanium. Because of this, arsenic-doped germanium is referred to be an n-type semiconductor.