Atomic Mass And Molecular Mass: Definition, Formula and Examples
Atomic mass and atomic weight are closely related and often used interchangeably but, they are not exactly the same thing. When we talk about mass of a single atom of an element it refers to Atomic mass while Atomic weight refers to the average mass of all naturally ocurring isotopes of an element, taking their abundance into account. Essentially, atomic weight is a weighted average of atomic masses.
This Story also Contains
- Atomic Mass And Molecular Mass And Dalton's Atomic Theory
- Some Solved Examples
- Practice more Questions from the link given below:
- Summary
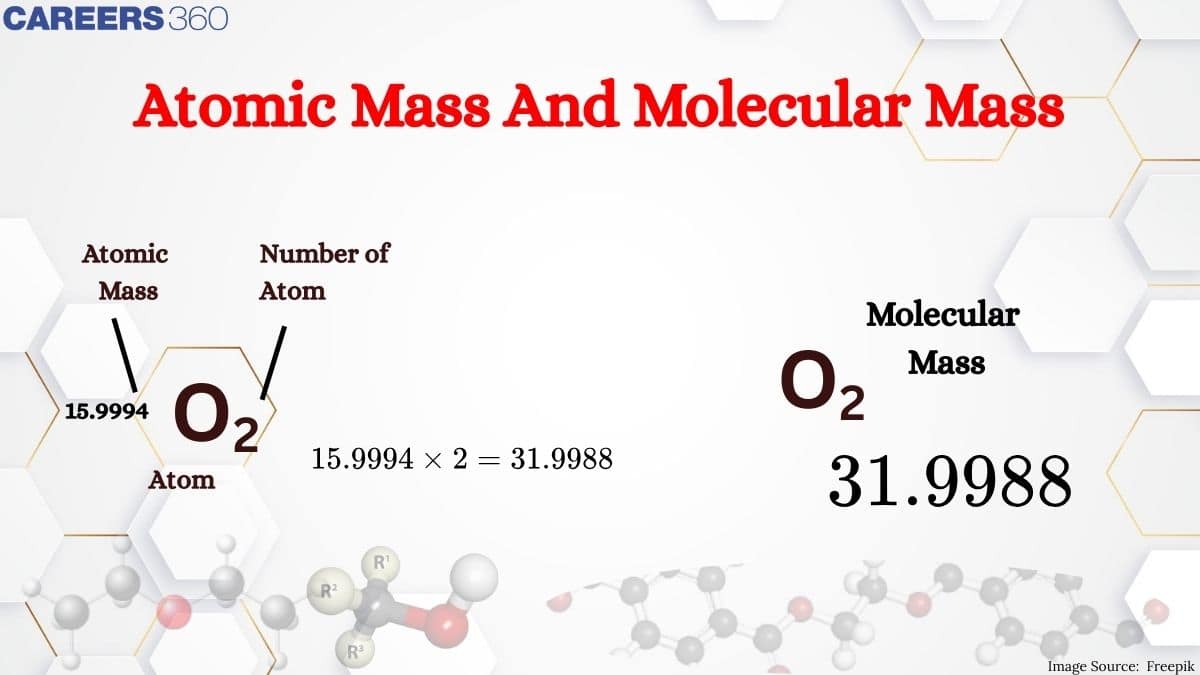
Molecular mass is the mass of molecules that are formed by the combination of elements. It is the sum of atomic masses of the elements present in a molecule. It is obtained by multiplying the atomic mass of each element by the number of its atoms and adding them together.
This theory was evolved from Dalton's work but further established by another scientist named Dmitri Mendeleev along with another chemist. The knowledge of molecular mass is important for calculating the stoichiometry of chemical reactions and also the properties of the substance.
Atomic Mass And Molecular Mass And Dalton's Atomic Theory
Atomic Mass :
One atomic mass unit is defined as a mass exactly equal to one-twelfth the mass of one carbon-12 atom.
And 1 amu = 1.66056 x 10-24 g
Mass of an atom of hydrogen = 1.6736 x 10-24 g
Thus, in terms of amu, the mass of hydrogen atom
=1.6736×10−24gm1.66056×10−24gm
= 1.0078 amu
= 1.0080 amu
Similarly, the mass of oxygen - 16 (16O) atom would be 15.995 amu.
Today, 'amu' has been replaced by 'u' which is known as unified mass.
When we use atomic masses of elements in calculations, we actually use average atomic masses of elements which are explained.
Average Atomic Mass :
Many naturally occurring elements exist as more than one isotope. When we take into account the existence of these isotopes and their relative abundance (percent occurrence), the average atomic mass of that element can be computed.
Average Atomic Mass =Σ( Mass of Isotopes )i×( \%abundance )i100
In the periodic table of elements, the atomic masses mentioned for different elements actually represented their average atomic masses.
Molecular Mass :
Molecular mass is the sum of the atomic masses of the elements present in a molecule. It is obtained by multiplying the atomic mass of each element by the number of its atoms and adding them together.
Formula Mass :

In case of solid compounds, formula of the compound does not represent its molecule, but only represents the ratio of different ions in the compounds. This is called a formula unit of the compound. In such compounds, therefore, we do not use the term molecular mass. Instead we use the term formula mass.
It may be noted that in sodium chloride, One Na+ is surrounded by six CI- and vice-versa. A formula such as NaCI is used to calculate the formula mass instead of molecular mass as in the solid-state sodium chloride does not exist as a single entity.
Formula mass of the compound is obtained by adding atomic masses of all the atoms in a formula unit of the compound. Thus, formula mass of sodium chloride = atomic mass of sodium + atomic mass of chlorine
= 23.0 u + 35.5 u = 58.5 u
Dalton's Atomic Theory
Some postulates of Dalton's Atomic Theory are:
-
Matter consists of indivisible atoms.
-
Atom is indivisible and cannot be broken down.
-
All the atoms of a given element have identical properties, including identical mass. Atoms of different elements differ in mass.
-
Compounds are formed when atoms of different elements combine in a fixed ratio.
-
Chemical reactions involve the reorganization of atoms. These are neither created nor destroyed in a chemical reaction.
Also Read:
Recommended topic video on (Atomic Mass And Molecular Mass )
Some Solved Examples
Example.1 What is the standard for the present system of atomic masses?
1) H - 1
2) C - 12
3) He - 4
4) O - 16
Solution
The present system of atomic mass is based on Carbon - 12 as the standard and has been agreed upon in 1961.
Hence, the answer is an option (2).
Example.2 What is the mass equivalent to 1 amu?
1) 112th of C-12 isotope
2) 116th of O-16 isotope
3) H-1 isotope
4) 14th of the He atom
Solution
1 amu = 112 thof the mass of C-12 atom.
Hence, the answer is option (1).
Example. 3 What is the mass (in u) of one Nitrogen atom in u if its mass in g is 2.32597×10−23 g?
1) 14
2) 15
3) 13.89
4) 14.007
Solution
As we learned in
Relation between amu and Gram -
12C is assigned a mass of exactly 12 atomic mass units (amu).
1 amu = 1.66056×10–24 g
mass in u=2.32597×10−23/1.66056×10−24=14.007u
Hence, the answer is option (4).
Example.4 What is the molecular mass of H2SO4?
(response should be like 67 or 70)
1) 96u
2) 100u
3) 102u
4) 98u
Solution
The molecular formula of H2SO4:
2H = 2u, S =32u, 4O = 64u
Thus, Molar mass of H2SO4 = 2 + 32 + 64 = 98u
Hence, the answer is option (4).
Example. 5 moles of AB2 weigh 125×10−3 kg and 10 moles of A2B2 weigh 300×10−3 kg The molar mass of A(MA) and molar mass of B(MB) in kgmol−1 are :
1)MA=50×10−3 and MB=25×10−3
2)MA=25×10−3 and MB=50×10−3
3)MA=5×10−3 and MB=10×10−3
4)MA=10×10−3 and MB=5×10−3
Solution
Let the atomic mass of A = x & atomic mass of B = y
∴ For AB2 :
5(x+2y)=125g⇒x+2y=25g⋯(I)
& For A2B2 :
10(2x+2y)=300g
⇒x+y=15g⋯⋯(II)
Solving equations (I) & (II), simultaneously, we get:
x=5×10−3 kg & y=10×10−3 kg
Thus, MA=5×10−3 kg
MB=10×10−3 kg
Hence, the answer is the option (3).
Practice more Questions from the link given below:
Summary
The atomic mass of an element is expressed relative to 12C isotope of carbon which has an exact value of 12. Usually, the atomic mass for an element is the average atomic mass obtained by taking into account the natural abundance of different isotopes of that element. The molecular mass of a molecule is obtained by taking sum of the atomic masses of different atoms present in a molecule. The molecular formula can be calculated by determining the mass percent of different elements present in a compound and its molecular mass or its vapour density.
