Atomic Radius in Periodic Table in Basic Chemistry - Overview, Example, Types & Trends, FAQs
Atomic size is defined as the distance between an atom's nucleus and its outermost shell. In basic chemistry, it is also denoted by the atomic radius which means the shortest distance between an atom's nucleus and its outermost shell. In a molecule, an atomic radius is half the distance between neighbouring atoms of the same element.
This Story also Contains
- Methods to Calculate Atomic Radius
- Types of Radius in Relation to the Different Types of Bonds
- Trends in the Periodic Table
- Some Solved Examples
As the size of an atom is of the order 1.2 ×10-10 m, measuring the atomic radii of chemical elements is a difficult undertaking. Because the electron cloud that forms an atom's shell has no fixed shape, determining the atomic size of an atom is challenging. As a result, we might conclude that determining the size of each particular atom is essentially impossible.
An element's atomic radius is a measurement of the size of its atoms, which is typically the mean distance between the nucleus centre and the boundary of the electrons' surrounding shells. Due to the lack of a well-defined border, multiple non-equivalent definitions of the atomic radius exist.
Methods to Calculate Atomic Radius
X-ray or other spectroscopic methods are used to determine an atom's atomic radius. The atomic radii of elements vary in a predictable fashion across the periodic table. The nuclear charge and energy level can be used to explain this tendency.
This is comparable to the concept of a circle's radius, in which the nucleus serves as the circle's centre and the electron's outermost orbital serves as the outside border of the circle.
According to the principle, the radius is derived from the distance between the nuclei of two bonded atoms. Atomic radii are thus determined by the bonds that they make. The radius of an atom varies depending on the bond it makes, hence there is no fixed radius for an atom.
Atomic Radii are classified as
-
Ionic radius
-
Covalent radius
-
Metallic radius
-
Van Der Waals radius.
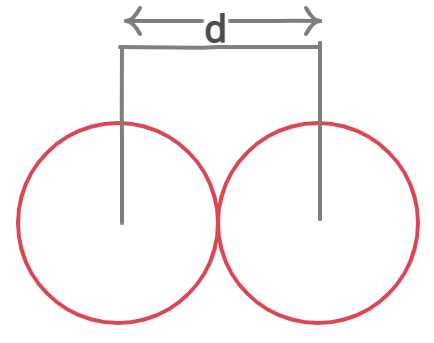
Atomic Radius r=d/2
d – Distance between the nuclei of two atoms.
Also read -
Types of Radius in Relation to the Different Types of Bonds
1. Van Der Waals Radius
- The Van Der Waals radius is a measurement of the size of an unbound atom, whether chemically, ionically, or covalently. It is defined as half of the distance between two equal and non-bonded atoms at their closest distance.
2. Ionic Radius
-
The radius of an atom creating an ionic bond or an ion is called the ionic radius.
-
Because the electrons and nucleus are constrained by atomic bonds, ions and atoms do not have a distinct shape.
-
The ionic radius is measured in Armstrong (A◦) or Pico meters (pm). The typical radius can be anywhere between 30 and 200 pm.
-
The ionic radius is not constant; it varies depending on the spin state of the electrons, the coordination number, and a variety of other factors.
-
The size of an ion increases as the number of coordinates increases. An ion with a high spin state of an electron has a larger ionic size than an ion with a low spin state of an electron.
-
If the charge of the ion is taken into account, the ion with a positive charge will be smaller than the ion with a negative charge.
3. Metallic Radius
- The metallic radius is the distance between two atoms connected by a metallic bond. In a metallic cluster, the metallic radius is half of the total distance between the nuclei of two adjacent atoms.
4. Covalent Radius
- An atom's covalent radius is defined as the radius of an atom that is in a covalent bond with another atom(s) of the same element.
- By comparing the bond lengths of two covalently bonded atoms of the same kind, the covalent radius of an atom may be calculated: if the two atoms are of the same kind, the covalent radius is just one half of the bond length.
-
While this is straightforward for some molecules, such as Cl2 and O2, in others, the covalent radius must be calculated by measuring bond distances to atoms whose radii are already known (for example, a C–X bond, where C's radius is known).
|
Related Topics |
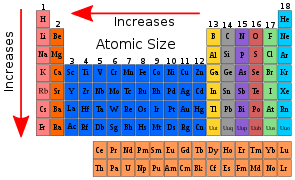
Trends in the Periodic Table
There are various trends which can be studied with respect to periods and groups in periodic table.
Across a Period
-
As we move from left to right in a period, the Covalent and Van der Waals radii fall as the atomic number increases. In a period, the alkali metals at the far left of the periodic chart have the largest size. The halogens on the periodic table's far right have the smallest size. Nitrogen has the smallest atomic size. After nitrogen, the atomic size of oxygen increases, then drops for fluorine. The atoms of inert gases are bigger than those of the halogens before them.
-
The nuclear charge grows by one unit in each subsequent element as we move from left to right in a period, while the number of shells remains constant. The electrons in all shells are drawn closer to the nucleus by the increased nuclear charge. As a result, each individual shell becomes smaller. As we move from left to right in a period, this causes the atomic radius to decrease.
-
As we progress from halogens to inert gases, the atomic radius abruptly increases. Because inert gases have entirely filled orbitals, this is the case. As a result, the inter-electronic is at its greatest. Because they do not form covalent bonds, we express atomic size in terms of Van der Waals radius. The radius of Van der Waals is bigger than the radius of covalent bonds. As a result, the atomic size of inert gas in a period is substantially larger than the halogen before it.
Down the Group
The atomic radii of elements rise as the atomic number in a group increases from top to bottom. The principal quantum number increases as we proceed down the group. At each subsequent element, a new energy shell is added. The valence electrons are moving away from the nucleus and away from the nucleus. As a result, the nucleus's attraction to the electron decreases. As a result, the atomic radius increases.
Some Solved Examples
Question 1: The incorrect decreasing order of atomic radii is :
1) $\mathrm{Mg}>\mathrm{Al}>\mathrm{C}>\mathrm{O}$
2) $\mathrm{Al}>\mathrm{B}>\mathrm{N}>\mathrm{F}$
3) (correct) $\mathrm{Be}>\mathrm{Mg}>\mathrm{Al}>\mathrm{Si}$
4) $\mathrm{Si}>\mathrm{P}>\mathrm{Cl}>\mathrm{F}$
Solution:
Correct order of atomic radii : $\mathrm{Be}<\mathrm{Mg}>\mathrm{Al}>\mathrm{Si}$
Hence, the correct answer is option (3).
Question 2: Which of the following has the highest atomic radius?
1) (correct) Rb
2) K
3) Na
4) Li
Solution:
The atomic radius increases on moving down the group. So Rb has the highest atomic radius among the given elements.
Hence, the answer is the option (1).
Question 3: The type of atomic radius which is defined for noble gases is
1) Covalent Radius
2) Ionic Radius
3) (correct) Van der Waal's Radius
4) Metallic Radius
Solution:
Since noble gases exist in non-bounded form, only Van der Waal's radius is defined for them.
Hence, the answer is the option (3).
Question 4: Which of the following arrangements represents the increasing order (smallest to largest) of ionic radii of the given species
$O^{2-}, S^{2-}, N^{3-} P^{3-} ?$
1) (correct) $O^{2-}<N^{3-}<S^{2-}<P^{3-}$
2) $O^{2-}<P^{3-}<N^{3-}<S^{2-}$
3) $N^{3-}<O^{2-}<P^{3-}<S^{2-}$
4) $N^{3-}<S^{2-}<O^{2-}<P^{3-}$
Solution:
The size of noble gas configuration anions increases down the group because of greater orbits and decreases along with the period because of greater nuclear charge.
$\mathrm{N}^{3-}>\mathrm{O}^{2-} \text { and } \mathrm{P}^{3-}>\mathrm{S}^{2-}$
also, $O^{2-}<S^{2-}$
$\therefore O^{2-}<N^{3-}<S^{2-}<P^{3-}$
Hence, the answer is the option (1).
Frequently Asked Questions (FAQs)
The atomic radius is defined as the smallest distance between the nucleus of an atom and its outermost shell.
Van der Waals Radius is half the distance between the nuclei of two similar non-bonded solitary atoms or two near identical atoms belonging to two neighbouring molecules of the same element.
The smallest element in the periodic table is Helium.
The ionic radius is the distance between the nucleus and the electron in the outermost shell of an ion.