Aufbau Principle - Definition, Example, Features, Configuration, FAQs
The Aufbau principle is a rule in chemistry that describes the order in which electrons fill the atomic orbitals in an atom. According to this principle, electrons occupy the lowest energy orbitals available before moving to higher energy orbitals. The word "Aufbau" means "building up" in German, reflecting the concept of filling orbitals in a stepwise manner. The order of filling generally follows this sequence: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p, and so forth. The Aufbau principle is foundational for understanding electron configurations and the structure of the periodic table and helps predict the arrangement of electrons in an atom's orbitals. Below is the depiction of electron filling order in various orbitals.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Aufbau principle diagram
- Aufbau Principle's Salient Features
- Aufbau Principle Exceptions
- Electronic Configuration using Aufbau principle
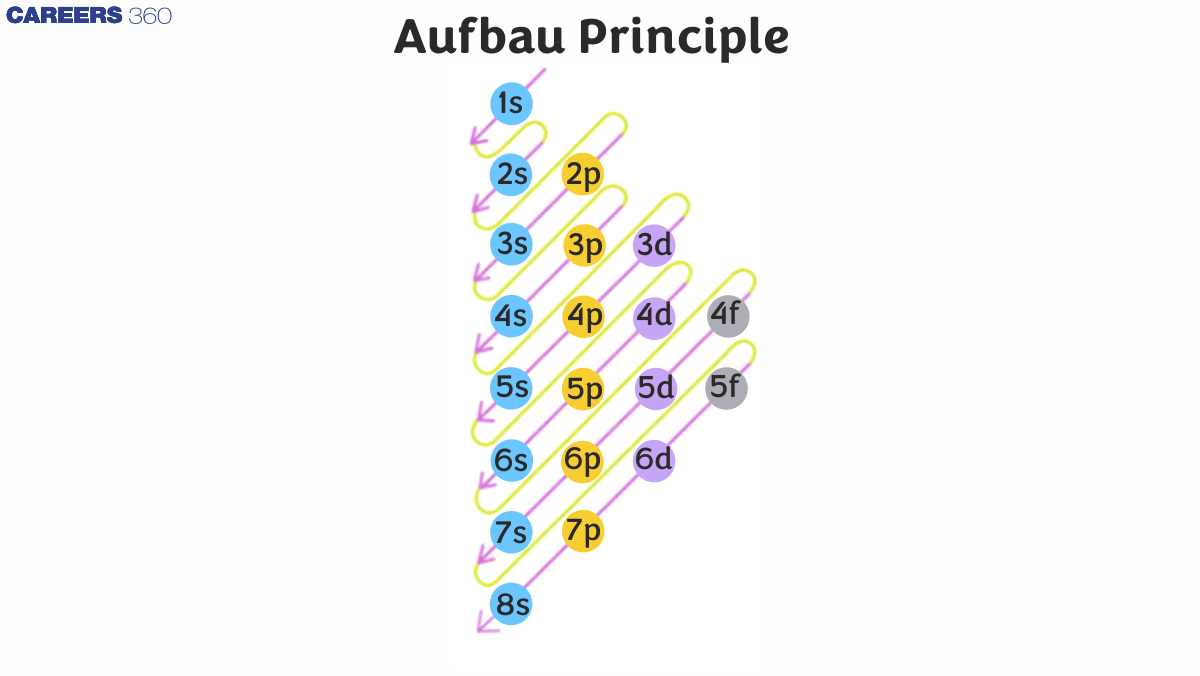
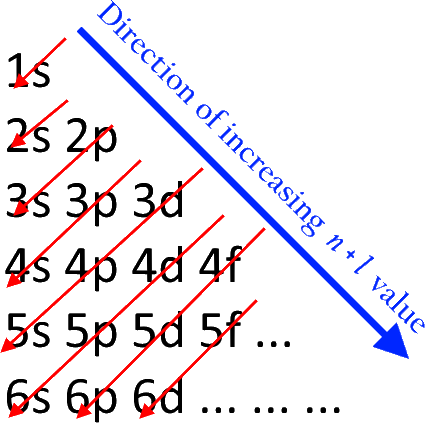
Aufbau principle diagram
Consider the electron arrangement of carbon, an atomic number six element. The 1s and 2s orbitals each have four electrons. The 2p subshell is occupied by the remaining two electrons. There is the option of pairing the electrons in one of the 2p orbitals or leaving them unpaired in two different but degenerate p orbitals. The orbitals are filled according to Hund's rule: for an atom with electrons in a set of degenerate orbitals, the lowest-energy configuration is the one with the most unpaired electrons. Thus, the n, l, and ms quantum numbers of the two electrons in the carbon 2p orbitals are identical, but their ml quantum numbers differ (in accordance with the Pauli exclusion principle). With an electron configuration of 1s22s22p2, the orbital diagram for carbon is:
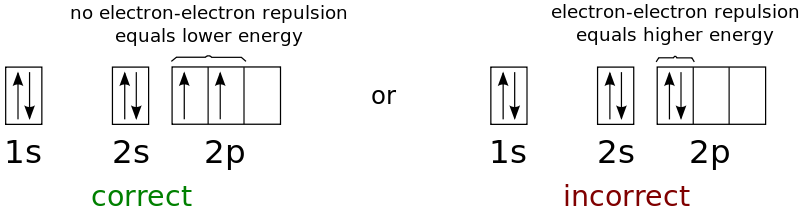
According to Hund's rule, nitrogen (atomic number 7) covers the 1s and 2s subshells and has one electron in each of the three 2p orbitals. The spins of these three electrons are unpaired. In any of the two 2p orbitals (the electrons have opposite spins), oxygen (atomic number 8) has a pair of electrons and a single electron in the other two. Fluorine (atomic number 9) contains only one unpaired electron in its 2p orbital. The noble gas neon (atomic number 10) has all of the electrons coupled, and all of the orbitals in the n = 1 and n = 2 shells are filled.
Also check-
Aufbau Principle's Salient Features
According to the Aufbau principle, electrons inhabit the lowest-energy orbitals first. This means that electrons can only enter higher-energy orbitals after lower-energy orbitals have been entirely filled.
The (n+l) rule may be used to establish the sequence in which the energy of orbitals grows, with the sum of the primary and azimuthal quantum numbers determining the orbital energy level.
Lower orbital energies correlate to lower (n+l) values. When two orbitals have the same (n+l) values, the orbital with the lower n value is said to have less energy.
The orbitals are filled with electrons in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Aufbau Principle Exceptions
Chromium's electron configuration is [Ar]3d54s1 not [Ar]3d44s2 (as suggested by the Aufbau principle). This is due to a number of variables, including half-filled subshells enhanced stability and the comparatively small energy gap between the 3d and 4s subshells.
Lower electron-electron repulsions in the orbitals of half-filled subshells increase stability through reducing electron-electron repulsions. Similarly, totally filled subshells improve the atom's stability. As a result, some atoms' electron configurations defy the Aufbau principle depending on the energy gap between the orbitals. Copper, for example, is an exception to this rule, having an electrical configuration that corresponds to [Ar]3d104s1 . The stability given by a totally filled 3d subshell explains this.
Related Topics |
Electronic Configuration using Aufbau principle
Electronic configuration of Sulphur
Sulphur has an atomic number of 16, indicating that it has a total of 16 electrons.
According to the Aufbau principle, two of these electrons are in the 1s subshell, eight are in the 2s and 2p subshells, and the rest are dispersed between the 3s and 3p subshells.
As a result, Sulphur’s electronic configuration can be expressed as 1s22s22p63s23p4.
Electronic configuration of Nitrogen
Nitrogen is a seven-electron element (since its atomic number is 7).
The 1s, 2s, and 2p orbitals are occupied with electrons.
Nitrogen's electronic configuration is written as 1s22s22p3.
Also read -
Frequently Asked Questions (FAQs)
The Aufbau Principle is a rule that states electrons fill atomic orbitals from the lowest to highest energy levels.
The general order of filling is as follows:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p, and so forth. This order follows the relative energy levels of each orbital.
The quantum numbers "n" and "l" in the (n + l) rule are used to specify the state of a specific electron orbital in an atom. Here, l denotes the angular momentum quantum number, which is related to the orbital's form.
It states that before any orbital in a subshell is doubly occupied, it is singly occupied with one electron, and all electrons in singly occupied orbitals have the same spin.
Also Read
06 Feb'25 11:21 PM
30 Dec'24 03:07 PM
20 Dec'24 11:57 PM
16 Dec'24 11:39 PM
16 Dec'24 11:27 PM
16 Dec'24 11:16 PM
21 Oct'24 04:01 PM
21 Oct'24 12:25 PM