Azeotrope - Definition, Types, Diagram Explanation, FAQs
Define Azeotropes.
A constant boiling point mixtures are also known as an azeotropic mixture is a type of mixture consists of two or more liquids whose proportions can not be altered or changed by simple distillation. The concept is that when we boils an azeotropic mixture, the vapour state contains the same amount of the constituents as that of unboiled mixture. Generally the composition of an azeotropic mixture will not be changed through while undergoing distillation, azeotropes are known as constant boiling point mixture.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Define Azeotropes.
- Q- Azeotropic Mixture Types ?
- Q- What is Constant Boiling Mixture?
- Q- What are Azeotropes Give an Example?
- Types of Azeotropes:-
- Q- Azeotrope phase diagram of Minimum - boiling or Positive azeotrope ?
- Here is the phase diagram of Maximum - boiling or Negative azeotrope
- Diagram Explanation :-
Q- Azeotropic Mixture Types ?
Minimum boiling azeotropic mixtures are the one that will boils at a temperature lower than the boiling point of other components in the pure state. The process of boiling and recondensation within a mixture of two solvents are due to the changes in chemical state. If the pressure will be held constant then the two variable parameters will be temperature and the composition. And, Maximum boiling azeotropes are those mixtures which shows the boiling point higher than any of its mixture constituents. In case of azeotrope the boiling point of the mixture will be higher than the constituents from which it is formed.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Q- What is Constant Boiling Mixture?
A constant boiling point mixtures are also known as an azeotropic mixture is a type of mixture consists of two or more liquids whose proportions can not be altered or changed by simple distillation. According to Raoult's law it is used to predicts the vapour pressures of an ideal mixtures with a function of composition ratio. In respect to Raoult's law the molecules that are present within azeotropic mixture will stick to each other at the same degree that they stick within themselves.
To understand we can take a look to this example, if the constituents of the mixture are X and Y, and X joins with Y with roughly equal energy as X joins with X and Y joins with Y or X – X & Y- Y. And thus this condition will be called as positive deviation from Raoult's law in which the constituents would have a disaffinity for each others as like X will sticks to X and Y will sticks to Y better than that of X sticks to Y. And the participants of the mixture that are having less total affinity within the molecules than of the pure constituents, they are more readily escape from the stuck i.e. together phase. And another case when X sticks to Y with aggressively force than that of X interact with X and Y interact with Y, then the result is known as a negative deviation according to Raoult's law.
In this case the molecules that are present within the mixture will interact more than that are present in the pure constituents, so that they need more energy to escape from the interaction i.e. liquid phase. And whenever the deviation may be able to cause a maximum or minimum change to its vapour pressure or composition function, then there will be a mathematical consequence that at that point, the vapour will constitute the same composition as that of the liquid, and thus resulting in an azeotrope.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 7 The P-block elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P-block elements
- NCERT notes Class 12 Chemistry Chapter 7 The P-block elements
Q- What are Azeotropes Give an Example?
A constant boiling point mixtures are also known as an azeotropic mixture is a type of mixture consists of two or more liquids whose proportions can not be altered or changed by simple distillation. When an azeotropic mixture will be boiled then the vapour state contains the same amount of the constituents as that of unboiled mixture.
In the case of mixtures of chemicals that have similar solvents for example n-hexane with n-heptane, form almost ideal mixtures that comes very close to follow Raoult's law. We can easily notice that whenever a mixture that will shows a positive deviation must have a point at which the tangent is horizontal, then the composition at that point will be in a positive azeotrope. At that point the total vapour pressure will be at its maxima.
Q- What are Azeotropes?
A constant boiling point mixtures are also known as an azeotropic mixture is a type of mixture consists of two or more liquids whose proportions can not be altered or changed by simple distillation
Types of Azeotropes:-
There are two types of azeotropic mixtures i.e. positive azeotrope or minimum boiling azeotrope and negative azeotrope or maximum boiling azeotrope. Minimum - boiling also known as Positive azeotrope . Minimum boiling azeotropes are those mixtures that will boils at a temperature lower than the boiling point of other components in the pure state. The process of boiling and recondensation within a mixture of two solvents are due to the changes in chemical state. If the pressure will be held constant then the two variable parameters will be temperature and the composition.
Q- Azeotrope phase diagram of Minimum - boiling or Positive azeotrope ?
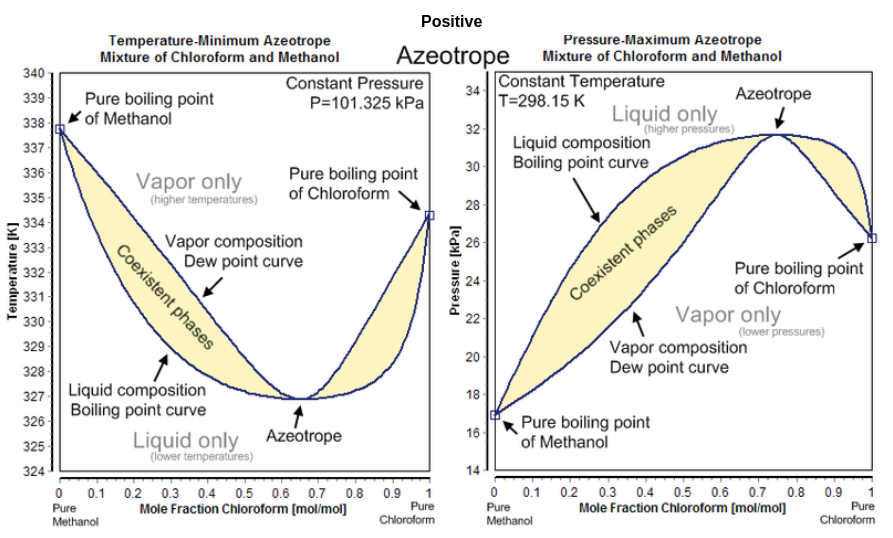
| Related topics , |
Maximum-boiling also known as Negative azeotrope
Maximum boiling azeotropes are those mixtures which shows the boiling point higher than any of its mixture constituents. In case of azeotrope the boiling point of the mixture will be higher than the constituents from which it is formed.
Here is the phase diagram of Maximum - boiling or Negative azeotrope
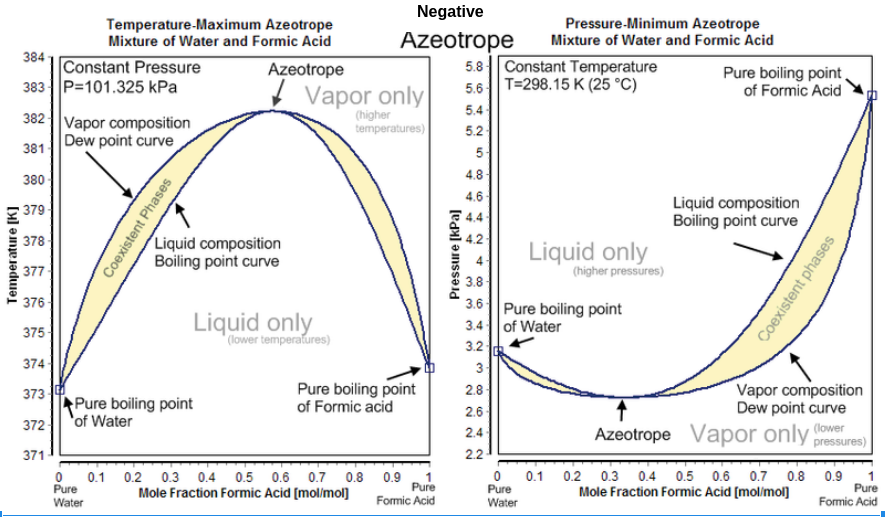
Diagram Explanation :-
Each azeotrope held a characteristic boiling point. The boiling point of an azeotrope mixture had two probabilities that it may be less than the boiling point temperature compared to the other constituents i.e. known as a positive azeotrope, or its boiling point of azeotropic mixture will be greater than the boiling point of any of its constituents i.e. known as a negative azeotrope.
When an boils an azeotropic mixtures, then the vapour content of the mixture will be in the same proportions to the constituents as that of unboiled mixture. Generally the composition of an azeotropic mixture will not be changed through while undergoing distillation, azeotropes are known as constant boiling point mixture.
A general example for a positive azeotrope is the mixture of ethanol and water. As we can observe that boiling point of Ethanol is 78.4 °C, and water boils at 100 °C, but the azeotropic mixture will boils at 78.2 °C, we can notice here that mixture has lower boiling point than either of its two constituents. And it shows that 78.2 °C is the minimum temperature at which any of the two constituent i.e. ethanol or water solution can boils at atmospheric pressure.
A positive azeotrope will be boils at a temperature that will be lower than any of the other boiling point ratio of its constituents. Positive azeotropes are also known as minimum boiling mixtures or pressure maximum azeotropes.
While a negative azeotrope must be boiled at a temperature that is higher compared to the other constituents. Negative azeotropes is referred to be maximum boiling mixtures or pressure minimum azeotropes. General example to understand negative azeotrope is that when hydrochloric acid and water forms azeotropic mixture.
To note here that boiling point of Hydrogen chloride is 84 °C and that of water is 100 °C, but the azeotropic mixture have the boiling point of 110 °C, which comprises higher than either of its two constituents. And it shows that, the maximum temperature at which any of the hydrochloric acid solution can boils will be 110 °C. Other examples:
Mixture of sulfuric acid and water
Mixture of nitric acid and water
Mixture of perchloric acid and water.
Mixture of hydrofluoric acid and water
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
There are two types of azeotropes. They are,
Minimum boiling azeotrope
Maximum boiling azeotrope.
The azeotrope mixture contains 95.63% ethanol and 4.37% water.
Yes, it is a mixture of compounds having the same composition in vapor and liquid state.
The two types of azeotropes are negative azeotrope and positive azeotropes.
Hydrochloric acid and water.
Also Read
19 Feb'25 11:05 AM
19 Feb'25 11:03 AM
16 Dec'24 11:40 PM
12 Dec'24 05:24 PM
12 Dec'24 12:58 PM
09 Dec'24 11:07 AM
09 Dec'24 11:06 AM
09 Dec'24 10:52 AM
13 Nov'24 07:10 PM