Benedicts Test - Overview, Preparation, Analysis, Limitations, FAQs
Have you ever wondered how we can test if a substance contains sugar? Why does a clear solution suddenly turn green, yellow, or red when heated with a certain chemical? And how can this test help us identify glucose or fructose in our food or even in urine? And how do we distinguish between reducing and non-reducing sugars in labs? For all these questions, we will read this article on Benedict's test.
This Story also Contains
- Reducing Sugar
- Reducing Sugar Test
- Benedict Test Reaction in the Benedict's Test for Urine Sample
- Principle of Benedict Test
- Benedict's Test Procedure: Preparation of Benedict's Reagent
- Benedict's Test for Reducing Sugars
- Analysis of Benedict's Test Results
- Limitations of Benedict's Test
- Some Solved Examples
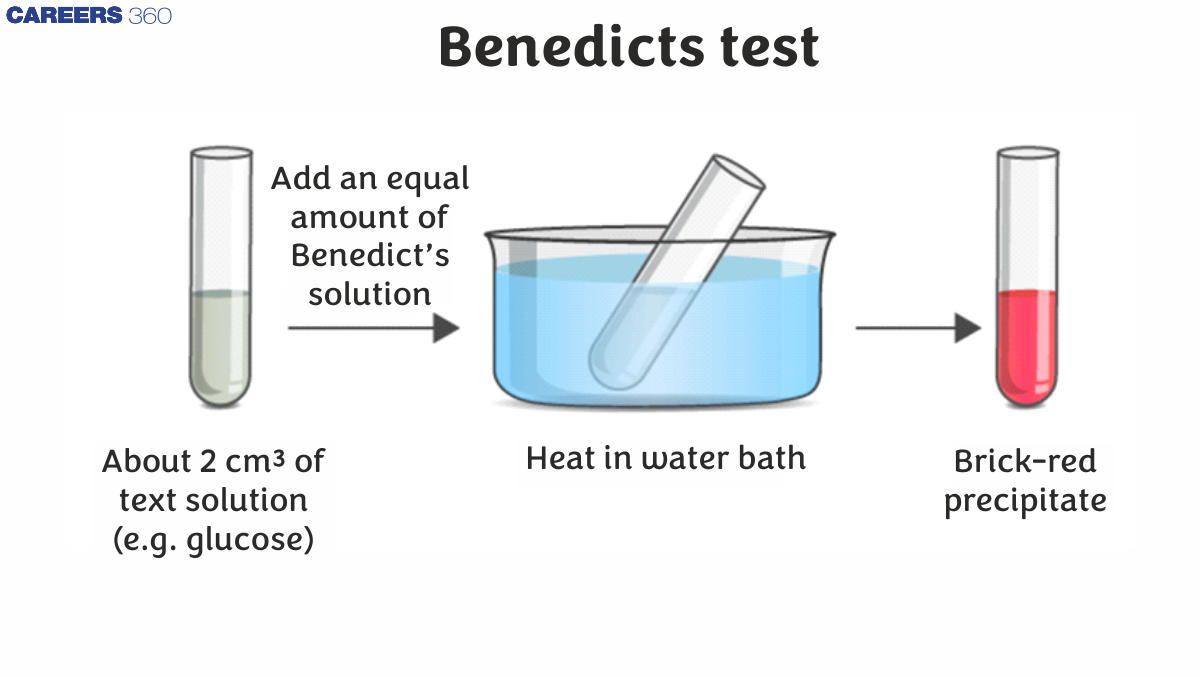
Benedict’s test is a chemical test that can be used to determine whether or not an analyte contains reducing sugars. As a result, this test can identify simple carbohydrates having a free ketone or aldehyde functional group. Benedict’s reagent (also known as Benedict's solution) is a complicated mixture of sodium citrate, sodium carbonate, and the pentahydrate of copper (II) sulphate, which is used in the test.
Reducing Sugar
A reducing sugar is any sugar (or carbohydrate) that has the ability to reduce other substances through the donation of electrons, and it contains a free aldehyde (-CHO) or ketone (-C=O) group. This means the sugar can donate electrons and act as a reducing agent in chemical reactions. The presence of a free aldehyde group (in glucose, fructose, etc.) or a ketone group (in fructose) allows these sugars to act as reducing agents.
Reducing Sugar Test
When Benedict’s reagent is subjected to reducing sugars, the reactions it undergoes result in the production of a brick-red precipitate, indicating a positive Benedict test principle. The changes in colour of Benedict’s reagent (from clear blue to brick-red) caused by exposure to reducing sugars are depicted in the figure below.
Benedict's sugar test is a straightforward method for determining the concentration of reducing monosaccharides in a solution. Benedict’s test can detect some disaccharides; however, sucrose (table sugar) is an unreactive disaccharide. Benedict’s solution is a reagent, a substance that changes colour when exposed to another substance.
Benedict Test Reaction in the Benedict's Test for Urine Sample
Benedict’s test can also be used to determine whether or not there is glucose in a Benedict test for a urine sample. When Benedict’s test for glucose is present in the analyte, the test produces a positive result because it identifies any aldehydes and hydroxy ketones, and glucose is an aldose whose open-chain form an aldehyde group in reducing substances in urine. The presence of ascorbic acid, homogentisic acid, and other reducing chemicals in the Benedict test for urine, on the other hand, can cause a positive reaction. As a result, a positive Benedict reaction test does not always mean the person is diabetic.
Chemical Reaction:
$
\mathrm{Cu}^{2+}+\text { Reducing Sugar } \rightarrow \mathrm{Cu}^{+}+\text {Reduction Products }
$
For glucose ( $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6$ ), the reaction can be represented as:
$
\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6 \text { (glucose) }+2 \mathrm{Cu}^{2+}+2 \mathrm{OH}^{-} \rightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6 \text { (oxidized) }+\mathrm{Cu}_2 \mathrm{O} \text { (copper(I) oxide) }+\mathrm{H}_2 \mathrm{O}
$
Principle of Benedict Test
A reducing sugar is transformed to an enediol formation when it is heated in the presence of an alkali (which is a relatively powerful reducing agent). The cupric ions (Cu2+) in Benedict’s reagent test are converted to cuprous ions (Cu+) when Benedict’s test for reducing sugars is present in the analyte. These cuprous ions combine with the reaction mixture to generate copper (I) oxide, which precipitates as a brick-red substance.
An Aldose + Benedict's reagent (blue) $\rightarrow$ Carboxylate ion + Brick Red precipitate
$
\mathrm{RCHO}+2 \mathrm{Cu}^{2+}(\text { citrate }) \rightarrow \mathrm{RCOO}^{-}+\mathrm{Cu}_2 \mathrm{~S}(\mathrm{~s})
$
Benedict reagent can be represented as:
$\mathrm{CuSO}_4($ Copper $($ II $)$ sulfate $)+\mathrm{Na}_2 \mathrm{CO}_3($ Sodium carbonate $)+\mathrm{Na}_3 \mathrm{C}_6 \mathrm{H}_5 \mathrm{O}_7($ Sodium citrate $)+$ Water $\left(\mathrm{H}_2 \mathrm{O}\right)$
|
Related Topics |
Benedict's Test Procedure: Preparation of Benedict's Reagent
In distilled water, combine 17.3 g of copper sulphate pentahydrate $\left(\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}\right), 100 \mathrm{~g}$ of sodium carbonate $\left(\mathrm{Na}_2 \mathrm{CO}_3\right)$, and 173 g of sodium citrate to make one litre of Benedict’s reagent (required quantity). Copper (II) sulphate serves as a source of Cu2+ ions, sodium carbonate serves as an alkaline medium, and sodium citrate creates Cu2+ ion complexes. As a solvent, distilled water is employed.
-
Alkalinity is imparted to the reaction media by anhydrous sodium carbonate.
-
Sodium Citrate - creates a compound with cupric ions, preventing them from decomposing into cuprous ions during storage.
-
The cupric ions are produced by copper (II) sulphate pentahydrate.
-
As a solvent, distilled water is employed.
By heating Benedict’s reagent in a test tube, you can determine its purity. The fact that the blue Benedict solution colour of the solution does not change when heated indicates that the reagent is pure.
Composition of Benedict reagent: $\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}+\mathrm{Na}_2 \mathrm{CO}_3+\mathrm{Na}_3 \mathrm{C}_6 \mathrm{H}_5$
Also read :
|
NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids | |
|
NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids | |
Benedict's Test for Reducing Sugars
One millilitre of analyte sample must be combined with two millilitres of Benedict’s reagent and heated for 3 to 5 minutes in a bath of boiling water. The formation of a brick-red coloured cuprous oxide precipitate confirms the presence of reducing sugars in the analyte.
Analysis of Benedict's Test Results
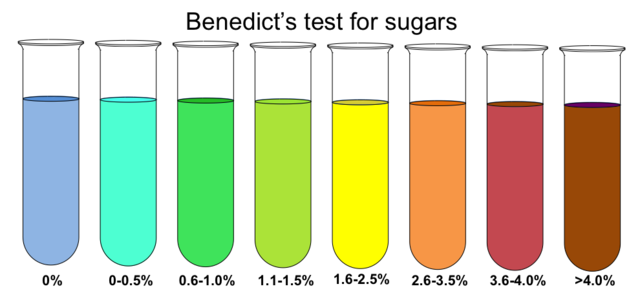
Depending on the amount of reducing sugar in the sample, the colour ranges from green to yellow to orange to brick-red; a sample containing 1% glucose produces a brick-red precipitate.
- Any mono or disaccharide having a hemiacetal or hemiketal group will change colour in Benedict's test.
- Because sucrose or table sugar lacks these groups, it will not result in a positive test.
|
OBSERVATION OF COLOUR |
% OF REDUCING SUGAR |
INTERPRETATION |
|
Blue or no colour change |
0% |
Absence of reducing sugar |
|
Green |
0.5-1 |
Trace amount of reducing sugar |
|
Yellow |
1-1.5 |
A small amount of reducing sugar |
|
Orange-Red |
1.5-2 |
Moderate amount of reducing sugar |
|
Brick Red |
>2 |
A large amount of reducing sugar |
Limitations of Benedict's Test
If some medicines are present, such as salicylates, isoniazid, streptomycin, penicillin, and p-amino salicylic acid, false-positive results in the test can occur. The compounds in the concentrated Benedict test for urine, such as urate, creatinine, and ascorbic acid, may diminish Benedict's reactivity (the reduction is slight).
Also read
Some Solved Examples
Question 1: Fructose and glucose can be distinguished by:
1) Benedict's test
2) Fehling's test
3) Barfoed's test
4) (correct) Seliwanoff's test
Solution:
Fructose/fruit sugar -
Structure is similar to Glucose, except it contains a ketonic functional group at carbon number 2.
Fructose and glucose can be distinguished by Seliwanoff's test
(1)Barfoed's test:- used for reducing sugar, both reduce sugar. So both give this test
(2)Benedict's test:- used for reducing sugar
$\therefore$ Both give this test
(3)Fehling's test:- Given by reducing sugars. So both give this test
.png)
Hence, the answer is option (4).
Question 2: A certain compound gives a negative test with ninhydrin and a positive test with Benedict's solution. The compound is:
1) Protein
2) (correct) Monosaccharide
3) Lipid
4) An amino acid
Solution:
A compound that gives a negative test with ninhydrin cannot be a protein or an amino acid. As it gives a positive test with Benedict's solution. So, it must be a monosaccharide but not a lipid.
Hence, the answer is option (2).
Question 3: Number of compounds from the following which will not produce orange-red precipitate with Benedict solution
is _______________
Glucose, maltose, sucrose, ribose, 2-deoxyribose, amylose, lactose
Solution:
The test uses Benedict's reagent, a mixture of copper sulfate, sodium citrate, and sodium carbonate, to identify reducing sugars. When reducing sugars are present, the reagent's copper ions are reduced to cuprous ions, which form a brick-red precipitate
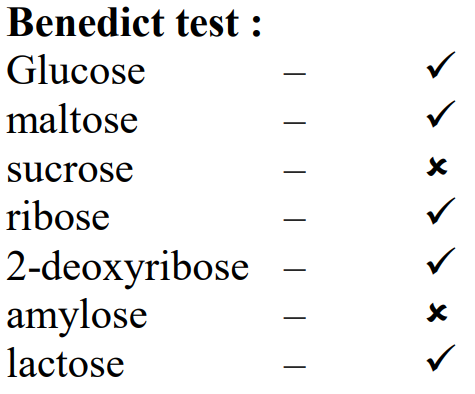
Neither Amylose nor Sucrose do not gives the Benedict‘s test.
Ans = 2
Hence, the answer is (2).
Question 4: Benedict’s reagent is used to detect the presence of:
A. Proteins
B. Reducing sugars
C. Amino acids
D. Lipids
Solution:
Benedict's reagent contains $\mathrm{Cu}^{2+}$ ions, which are reduced to $\mathrm{Cu}_2 \mathrm{O}$ (brick-red precipitate) by reducing sugars (glucose, fructose, lactose, maltose).
So it is a test for reducing sugars.
Hence, the correct answer is option (B)
Question 5: Which structural feature makes a sugar give a positive Benedict’s test?
A. Presence of more than one –OH group
B. Presence of a free aldehyde/ketone group
C. Ring structure
D. Long carbon chain
Solution:
Reducing sugars must have a free anomeric carbon that can open into an aldehyde or ketone $\rightarrow$ reduces $\mathrm{Cu}^{2+}$ ions.
Hence, the correct answer is option (B)
Practice More Questions With the Link Given Below
| Tests for Carbohydrates practice questions |
| Classification of Carbohydrates and its Structure practice questions |
Frequently Asked Questions (FAQs)
Benedict test is used for the detection of the presence of simple benedict’s test for carbohydrates in an unidentified analyte. This test can be used to look for free aldehyde or ketone functional groups in reducing sugars. A monosaccharide or a disaccharide might be used as the reducing sugar.
Benedict’s test is performed by mixing one millilitre of analyte solution with two millilitres of Benedict’s reagent in a test tube. Then, for about 3 minutes, this combination must be heated in a hot water bath (or until a visible change in colour occurs).
Benedict’s reagent, also called Benedict’s solution test, is a chemical reagent made composed of a complicated mixture of sodium citrate, sodium carbonate, and copper (II) sulphate pentahydrate. Benedict’s reagent changes colour from clear blue to brick red when exposed to reducing sugars and other reducing chemicals.
Glucose, Fructose, Ribose are examples of substances that give positive results for Benedict’s test.
A special reagent called Benedict's solution can be used to test for simple carbohydrates, such as glucose. Normally blue, Benedict's solution changes color if simple carbohydrates are present—turning green or yellow for a low concentration and red for a high concentration.
