Bohr's Model Of An Atom
Danish physicist Niels Bohr in the early years of the twentieth century endeavour to develop the progressive atom theory that not only revolutionized the outlook on atomic form but also formed the new quantum theories of modern times. Bohr’s version succeeded in overcoming the limitation of classical physics regarding the stability of atoms and the inherent line atomic spectra. At the center of Bohr’s model are several postulates suggested, in addition to the traditional tenets of classical mechanics.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Bohr's Model and its Postulates
- Solved Examples Based On (Bohr's Postulate)-
- Conclusion
In this article, we will cover the concept of Bohr's model and its postulates. This concept falls under the broader category of Atomic structure, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Bohr's Model and its Postulates
Bohr’s model was proposed to be an improvement of the planetary model and was for the same reason developed; it included the electrons circling in orbits around the nucleus and in these orbits, they were only allowed to exist in certain fixed and discrete energy levels as opposed to the planetary model which predicted that electrons would move in free and smooth paths like planets around a star. These levels are quantized in the sense that they have been allocated specific values and an electron can only transfer from one level to another in quanta of energy equal to the difference between the two levels.
Bohr’s model also gave some explanation for the phenomenon that is known as stationary states, whereby the electrons in their stable orbits do not radiate as perceived by classical theories. This model was able to thoroughly account for the spectrum of hydrogen and also paved the way for something that turned out to be quantum mechanics. Even though there are significant weaknesses in Bohr’s model like the inability to explain the phenomena of multielectron atoms, this model is considered one of the important steps in the development of atomic theory as it gives a proper understanding of the electron behavior in an atom.
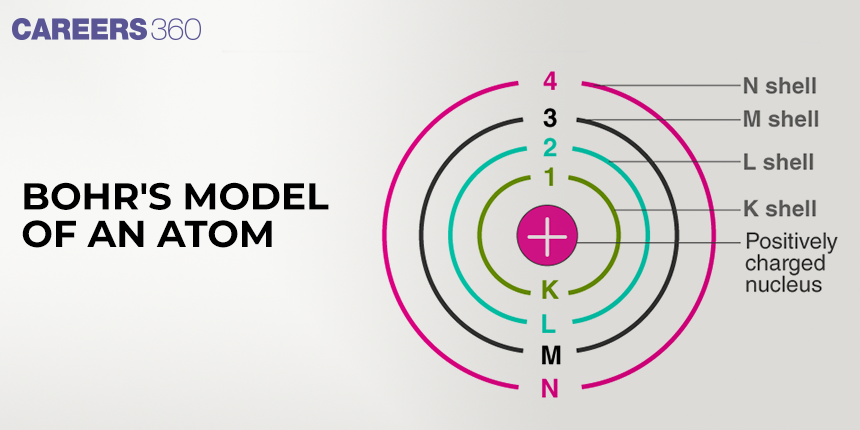
1. The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states, or allowed energy states and are arranged concentrically around the nucleus. The force of attraction between the nucleus and an electron provides the centripetal force required by the electron to carry out the circular motion.
2. The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when the required amount of energy is absorbed by the electron or energy is emitted when an electron moves from a higher stationary state to a lower stationary state
3. Energy can be absorbed or emitted when an electron transitions between two different orbits, and the frequency of the photon involved can be calculated using the formula:
|E1−E2|=hν
4. The angular momentum of an electron is quantized. In a given stationary state, it can be expressed as
L= mvr= nh/2$\pi$, n = orbit number
So only those energy states (or orbits) are allowed in which the above equation holds for the angular momentum.
Note: Bohr's model is only valid for Hydrogen species or electronic species that contain only a single electron.
Recommended topic video on (Bohr's Model Of An Atom )
Solved Examples Based On (Bohr's Postulate)-
Example 1: Which of the following statements is incorrect for Bohr's model of an atom?
1) (correct) It is valid for a multi-electronic species
2) The angular momentum of an electron is quantized
3) The centripetal force of attraction required for circular motion is provided by the electrostatic force of attraction between the electron and the nucleus
4) Orbits have fixed energy and are referred to as stationary states
Solution:
Bohr's model is valid for only uni-electronic species.
All other given statements given in the options are correct.
Hence, the answer is the option (1).
Example 2:
Which of the following is incorrect for Bohr's model of an atom?
1) (correct) It is valid for multi-electronic species.
2) The angular momentum of an electron is quantized.
3) The centripetal force of attraction required for circular motion is provided by the electrostatic force of attraction between the electron and the nucleus.
4) Orbits have fixed energy and are referred to as stationary energy.
Solution
Bohr's model is valid for electronic species only.
Hence, the answer is the option (1).
Conclusion
The version that was presented by Bohr was able to explain the spectral traces of hydrogen. Bohr’s model of the atom can be viewed as a scientifically critical stage in the paradigm shift from the old paradigm of classical physics to the new approaches of the modern quantum mechanics paradigm. While Bohr’s model had some problems, such as its inability to deliver a qualitative description of multielectron atoms and the quantization of angular momentum, it was useful in developing an early atomic model that helped accurately describe atomic spectra.
Also, check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Frequently Asked Questions (FAQs)
According to Bohr, the electrons reside in a specified region in a circle which is stable and hence can be referred to as stationary circular orbits because they do not emit energy due to centripetal force balancing the attractive force of the nucleus.
From emission and absorption spectra, discrete electron transition.
As Bohr's model applies to Hydrogen atoms only, that's why this model couldn't explain the multielectron atoms.
The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states, or allowed energy states and are arranged concentrically around the nucleus. The force of attraction between the nucleus and an electron provides the centripetal force required by the electron to carry out the circular motion.
Also Read
06 Feb'25 11:29 PM
06 Feb'25 11:19 PM
20 Oct'24 05:49 PM
30 Sep'24 12:12 PM
30 Sep'24 11:43 AM
30 Sep'24 11:16 AM
18 Sep'24 10:15 AM
16 Sep'24 03:04 PM