Calcium Hydroxide - , Toxicity, Properties, Structure, Uses, FAQs
In chemistry, calcium hydroxide common name is called slaked lime. Calcium hydroxide is a colorless crystal or white powder. The question arises, “What is the formula of calcium hydroxide?”. The calcium hydroxide formula or chemical formula of calcium hydroxide is Ca(OH)2. It is given the name slaked lime because it is produced when quick lime is mixed with water. Ca(OH)2 chemical name Calcium hydroxide is also produced by the reaction of sodium hydroxide with calcium chloride that is dissolved in water.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Properties of Calcium Hydroxide
- Toxicity of Calcium Hydroxide
- Calcium Hydroxide Uses or Slaked Lime Use
- Structure of Calcium Hydroxide
- The Acidity of Calcium Hydroxide
Calcium hydroxide also has some other names hydrated lime, builders lime, slaked lime, pickling lime, etc. The solubility of calcium hydroxide is low it is dissociated in water forming OH- ions which indicate that it is basic. The saturated solution or aqueous solution of calcium hydroxide is called lime water. The dissociation of calcium hydroxide in water is shown below.
Ca(OH)2+H2OCa2+(aq)+2 OH-
An appropriate temperature lime dissolves in water to make an alkaline solution with a pH of about 12.5. At high pH values, the hydroxide anion is already present in it so due to the common ion effect the solubility of calcium hydroxide is reduced. When lime is dissolved in water only a touch portion of it dissolves in water and forms lime water and therefore the remaining portion that is not dissolved remains as a suspension and is named as milk of lime. This lime water can also react with acids and thereby forming a salt of calcium hydroxide. Lime water also dissolves some metals like aluminum too. Calcium hydroxide contains two groups so it is a moderate base and its contact with the skin can cause burn also.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Properties of Calcium Hydroxide
Calcium hydroxide is also known as slaked lime. Calcium hydroxide is a soft, crystalline, white powdery material with a bitter taste. The molecular weight of calcium hydroxide is 74.093 grams per mole with a density of 2.211 grams per centimeter cube. 853K is the melting point of calcium hydroxide. The solubility of calcium hydroxide with water is low and it also reduces on increasing temperature, the solubility product is 5.5×10-6. But its solubility with glycerol and acids is quite high. The reaction of calcium hydroxide with carbon dioxide is called carbonation and its results in the formation of calcium carbonate CaCO3.
Toxicity of Calcium Hydroxide
When calcium hydroxide contact with the eye and skin it will cause irritation and burn the skin and consequently eye damage will occurs. Breathing of calcium hydroxide cause irritation to the nose, throat, and lungs thereby causing coughing, wheezing, and breathing problems. When someone breaths calcium hydroxide it will cause problems such as heart disease, lung cancer, respiratory problems, and emphysema. So care must be given while handling with calcium hydroxide when it comes in contact with the skin washing immediately can reduce the effect of calcium hydroxide.
Also read :
- NCERT notes Class 12 Chemistry Chapter 8 The d and f block elements
- NCERT solutions for Class 12 Chemistry Chapter 8 The d and f block elements
- NCERT Exemplar Class 12 Chemistry solutions Chapter 8 The d and f block elements
Calcium Hydroxide Uses or Slaked Lime Use
Calcium hydroxide is used in sewage treatment as a clarifying agent.
It is also used in the paper industry for the conversion of wood to wood pulp.
Calcium hydroxide is a very important component for the production of ammonia.
As calcium hydroxide is basic so it is used as a pH modifier.
For the production of plastics, calcium hydroxide is an important component.
It is also present in hair care products.
It is used in the production of pesticides and for the manufacture of ebonite.
In the processing of sugarcane, a carbonation reaction is used so it involves the use of calcium hydroxide.
It is used in the leather industry for the separation of hair or fur from animals.
It also has applications in the medical field mainly the dental field. That is the filings used for the process of root canal treatment contain calcium hydroxide.
Due to the high pH value of calcium hydroxide, it is used in fresh water treatment for raising the pH value. This is also due to its low toxicity in water and also self-regulating property and it will not raise the pH of water too much.
As calcium hydroxide is less toxic it has many applications in the food industry too. Mainly for the pickling of cucumber and other foods.
Due to the reaction of calcium hydroxide with carbon dioxide, it is used for the removal of carbon dioxide.
Related Topics link, |
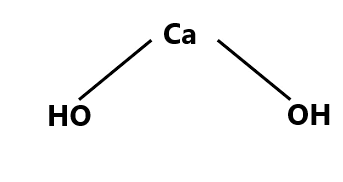
Structure of Calcium Hydroxide
Just like all the metal hydroxide calcium hydroxide also adopts a polymeric structure. The structure of Calcium hydroxide is similar to that of magnesium hydroxide. The bond present in the Calcium hydroxide is ionic as the calcium and the hydroxide are existing in their ionic forms. Ionic bonds exist between Ca2+ and OH- ions. And the calcium metal ion is bonded to two hydroxide ions.
Since the valency of calcium is two plus that it requires two more electrons to complete its octet that is why it directly forms a bond with two hydroxide ions. Calcium hydroxide exists in its polymeric structure in the solid form and this polymeric structure is facilitated by the hydrogen bonding in between the molecules. The structure of calcium hydroxide is shown below.
Calcium hydroxide reaction with water
Ca(OH)2+H2OCa2+(aq)+2 OH-
Preparation of calcium hydroxide
Calcium hydroxide is found naturally but it is not present in excess so the preparation of calcium hydroxide is necessary. Calcium hydroxide is mainly prepared commercially by the treatment of calcium oxide with water. The following is the reaction taking place on this.
CaO+H2O→Ca(OH)2
Calcium hydroxide is also prepared in the laboratory by the reaction of calcium chloride with sodium hydroxide with NaCl as a byproduct. The following is the reaction taking place on this.
CaCl2+NaOH→Ca(OH)2+NaCl
A white precipitate of Calcium hydroxide is obtained after the reaction.
NCERT Chemistry Notes:
The Acidity of Calcium Hydroxide
Calcium hydroxide is a compound containing two hydroxyl groups so it gives two hydroxyl ions per molecule upon dissociation it can be regarded as a di acidic molecule. Hence the acidity of Calcium hydroxide is 2. The dissociation of calcium hydroxide can be shown below.
Ca(OH)2Ca2++2OH-
This two hydroxyl group is responsible for the acidity of the medium.
Also check-
Frequently Asked Questions (FAQs)
The common name of calcium hydroxide is slaked lime.
Calcium hydroxide.
The metal present in calcium hydroxide is Ca.
Ca(OH)2 is slightly soluble in water and its solubility decrease with increasing temperature.
Calcium hydroxide.
Ca(OH)2 is a strong base because it dissociates completely into its respective ions. The calcium hydroxide dissociates in the following way.
Ca(OH)2Ca2++2OH-
Calcium hydroxide is also known as slaked lime, Ca(OH)2 and is obtained by the reaction of water with calcium oxide.
It is a white-colored powdery compound.
Calcium hydroxide contains two hydroxyl groups that is Ca(OH)2 and it dissociates completely in water to its respective ions.
Ca(OH)2
The pH of Ca(OH)2 is 12.8 and indicates that it is a base.
Also Read
11 Mar'25 07:18 PM
11 Mar'25 07:09 PM
17 Oct'24 05:14 PM
17 Oct'24 04:50 PM
30 Sep'24 11:29 AM
30 Sep'24 08:51 AM
21 Jul'22 03:42 PM
21 Jul'22 02:44 PM
20 Jul'22 05:01 PM