Calcium Sulphate - Definition, Structure, Uses, FAQs
Calcium sulphate is a naturally occurring inorganic calcium compound with the chemical formula CaSO4 and its related hydrates. It is usually known in its dihydrate state, CaSO4. 2H2O. It is a white coloured powder which is barely soluble in water. Calcium sulphate consists of two well-known hydrate forms; one generally known as Plaster of Paris and the other is a naturally occurring mineral Gypsum. It has many industrial uses. They are largely used in many fields especially medical and construction. Calcium sulphate produces permanent hardness of water.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Structure of calcium sulphate
- Calcium sulphate Formula mass
- Uses of Calcium Sulphate
Structure of calcium sulphate
Calcium sulphate consists of one calcium atom, one sulphur atom and four oxygen atoms. It is an ionic compound. It contains one cation of calcium and one anion of sulphate. Calcium ion shows +2 valency while polyatomic sulphate ion shows -2 valency. So combining these it forms a neutral compound of calcium sulphate, CaSO4.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Hydration states of Calcium Sulphate
Calcium sulphate exists in three hydration states.
i) Anhydrous state: CaSO4
ii) Dihydrate state: CaSO4 · 2 H2O
It is also named as gypsum. CaSO4 · 2 H2O chemical name is calcium sulphate dihydrate.
iii) Hemihydrate state: CaSO4 · 1⁄2 H2O is the hemihydrate formula
The commercial name of calcium sulphate hemihydrate is Plaster of Paris. Sometimes calcium sulphate hemihydrates are distinguished as α- hemihydrate and β-hemihydrate.
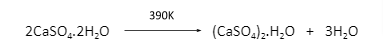
Plaster of Paris (2CaSO4.H2O)
It is abbreviated as POP. It is a white powder. It is the hydrated calcium sulphate salt and on mixing with water it changes into gypsum. The chemical name is calcium sulphate hemihydrate. Heating at 393 K gives gypsum. If is heated in high temperature; higher than 393 K it will form anhydrite which is also known as ‘dead burnt plaster’ which on again mixing with water gives gypsum.
Gypsum
Gypsum is a very common mineral composed of calcium sulphate dihydrate having chemical formula CaSO4·2H2O and molecular mass of 172.17. Pure gypsum is white in colour while other impurities might give a range of colours. It is used as a fertilizer, soil conditioner, gypsum blocks, and gypsum mortar and as the major component in different forms of plaster, blackboard, and sheet rock or drywall. Gypsum are used for centuries for sculpture, statuary, carvings, and other ornaments.
Solubility
Calcium sulphate solubility is least in water and after solidification it does not dissolve readily with water. In water different crystalline phases of calcium sulphate undergo dissolution and it is exothermic. In calcium sulphate its solubility decreases as temperature increases and solubility increases as temperature decreases.
It’s having retrograde solubility. Some calcium compound such as calcium hydroxide also undergo retrograde solubility, it’s exothermic and releases heat in dissolution reaction. So it is required to decrease temperature of the solution close to its freezing point to get maximum dissolution of calcium sulphate or calcium hydroxide.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 10 The S-Block Elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 The S-Block Elements
- NCERT notes Class 12 Chemistry Chapter 10 The S-Block Elements
Calcium sulphate Formula mass
Molecular weight calculation;
40.078 + 32.065 + (15.9994*4)
Molecular weight= 136.14 g/mol
Preparation
Calcium sulphate prepared in laboratory by addition of dil. H2S04 to CaCl2 solution.
CaCl2 + H2SO4 - CaSO4 + 2HCl
Many chemical processes give calcium sulphate as by-product for example;
Gases from fossil fuel power plants, cement manufacture and other process contain sulphur dioxide. These exhaust gases are scrubbed to decrease the sulphur oxide content by using limestone or lime. In this flue-gas desulfurization process impure calcium sulphite is produced and then it oxidises on storage to give calcium sulphate, calcium sulphate precipitate are formed in phosphoric acid production reaction from phosphate rock, calcium fluoride reacted with sulphuric acid in hydrogen fluoride production also gives calcium sulphate precipitate and calcium sulphate by-products are also produced in the process zinc refining.
| Related topics, |
Uses of Calcium Sulphate
- Calcium sulphate is mainly used for plaster of Paris and stucco production.
- In food industry calcium sulphate is used mainly in making of bread, cookies and brownies. Calcium sulphate serves several functions in baked goods. In baked goods calcium sulphate have different uses as anticaking agent, aid in the preservation of colour in coatings, texturizer, and flour bleaching agent, pH regulator, stabilizer and thickener. Calcium sulphate hydrate utilized as coagulant in some food products such as bean curd. It is permitted in some cheese Products, Bakery Products; Frozen Desserts, Artificial Sweeteners for Jelly and Preserves; Condiment Vegetables and some candies.
- In dentistry calcium sulphate is used for the regeneration of bones as a graft material and extender and it act like barrier in particular guided tissue regeneration. It is an oddly biocompatible material and fully resorbed following implantation. It does not evoke a notable host response and make a calcium-rich milieu in particular implanted area. Calcium sulphate is a very common compound having application in several medical and dental procedures. It was used to help treat different types of osseous defects by acting as a bone substitute. It have different therapeutic applications because of its unique property. In dentistry, calcium sulphate gives good results in periodontal infrabony defects. Its restorable and biocompatible specialities utilized in ridge and sinus augmentations, furcation, periodontal intrabony and periapical defects, management of osteomyelitis and even during dental implant placement.
- Drierite in its anhydrous state seems to be in blue or pink colour. It is because of the impregnation with cobalt (II) chloride that acts as the moisture indicator.
- Calcium sulphate is an excellent soil conditioner and it utilized to enhance soil structure. It provides the needed calcium and sulphur. It has nutrient value. Calcium enhances soil structure and generate oxygen conditions needed for the soil and plants health. This improves the crop quality, hence, enhance yields. Plants are showing more sulphur deficiencies because of reduced levels of sulphur available in the atmosphere. Gypsum is an excellent source of sulphur for nutrition of plants. Calcium and sulphur useful for neutralizing soil activity.
- Gypsum converted to calcium sulphate hemihydrate by heating. This has the molecular formula CaSO4 .1/2 H2O. 100-150 °C temperature are needed to eliminate the water from its structure. Temperatures of 170 °C are used for industrial calcination, but γ-anhydrite starts to generate at these temperature. The heat energy applied to the gypsum tends to eliminate water as vapour. Partial dehydration is take place here;
CaSO4.2 H2O → CaSO4.1/2 H2O + 1/2 H2O↑
This reaction is endothermic, so it is relevant to the drywall performance, nonflammable to residential structures. In a fire, drywall preventing or substantially retarding damage to the framing and consequent structural collapse because part behind the drywall sheet remain cool as water eliminates from gypsum. But calcium sulphate release oxygen and act as oxidizing agent at higher temperature. Calcined gypsum rehydrated by mixing with water at ambient temperatures, it chemically revert to the dihydrate form very quickly, while physically "setting" to form a strong and rigid gypsum crystal lattice;
CaSO4.1/2 H2O + 1/2 H2O↑ → CaSO4 . 2 H2O
This is an exothermic reaction and is responsible for the utilization of gypsum to make drywall, sticks for blackboard chalk and other different shapes. Also moulds for metal casting. The dehydration conditions are altered to obtain the α- and β-hemihydrate by adjusting the porosity of the hemihydrate. On heating about 180 °C temperature the nearly water-free form called γ-anhydrite is produced.
Dihydrate state reached when γ-Anhydrite reacts slowly with water. β-Anhydrite is generated on heating above temperature of 250 °C. Natural anhydrite are unreactive with water, besides fine ground. The similar crystal structures having "channels" that can accommodate different amounts of water or other smaller molecules such as methanol is the reason for variable composition of hemihydrate and γ-anhydrite and also their easiness for inter-conversion.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Calcium sulphate are compounds which exists in three different hydration states that includes anhydrous state (CaSO4), dihydrate state (CaSO4 · 2 H2O) it is also called gypsum and hemihydrate state (CaSO4 · 1⁄2 H2O) It is also known as Plaster of Paris. Sometimes hemihydrates are distinguished as α- hemihydrate and β-hemihydrate.
Gypsum is a compound which slows down the setting of cement.
The molecular formula of plaster of Paris is 2Caso4.H2O
Calcium sulphate prepared in laboratory by addition of dilute sulphuric acid to CaCl2 solution. Its chemical reaction is
CaCl2 + H2SO4 - CaSO4 + 2HCl
The 2h2Ochemical name is hydrogen peroxide.
CaSO4 chemical name is Calcium sulphate.
Calcium sulphide formula is CaS.
Also Read
19 Feb'25 10:44 AM
17 Dec'24 10:24 AM
17 Dec'24 10:21 AM
29 Nov'24 12:13 PM
30 Sep'24 02:31 PM
30 Sep'24 11:46 AM
26 Sep'24 06:10 PM
26 Sep'24 05:53 PM