Potassium Hydroxide - Formula, Uses, Properties, Structure, FAQs
Potassium Hydroxide
Potassium hydroxide, also known as caustic potash, is an inorganic substance and potassium hydroxide formula or caustic potash formula is KOH. Caustic potash, lye, and potash lye are all names for potassium hydroxide. This base is made up of alkali metal hydroxide, which is a very potent alkali metal. It has the appearance of a transparent solution in its aqueous state. KOH comes in a variety of solid forms, including white to slightly yellow lumps, flakes, pellets, and rods. In its solid state, this chemical does not have a distinctive odour. KOH chemical name is potassium hydroxide.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Potassium Hydroxide
- Properties of Potassium Hydroxide
- Structure of Potassium Hydroxide
- Solubility of Potassium Hydroxide
- Preparation of Potassium Hydroxide
- Potassium Hydroxide Uses:
- Chemical Properties of Potassium Hydroxide
- Health Hazards of Potassium Hydroxide
Caustic potash is another name for potassium hydroxide.
It is an inorganic substance.
The chemical formula of potassium hydroxide is KOH.
KOH has a molecular weight/molar mass of 56.11 g/mol.
It is non-flammable but quite corrosive.
Chemical manufacture, cleaning compounds, and petroleum refining all use it.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
It also has a caustic tendency, which means it causes a burning feeling and is irritating to the skin when exposed to it. It is recommended that it be used and handled with care. It is also a very caustic substance. It has the ability to corrode metals as well as living cells and tissues. Potassium hydroxide is an alkali metal hydroxide. As a result, it is a strong base. It is, in fact, one of the most stable bases. At standard conditions, the pH of a 1 mol/L KOH solution is 10.98, which is quite high.

Pellets of Potassium Hydroxide
Properties of Potassium Hydroxide
KOH | Potassium hydroxide |
Molecular Mass | 56.11 g/mol |
Density | 2.044 g/cm3 |
Boiling point | 1327 °C |
Melting Point | 360 °C |
Also read :
- NCERT notes Class 11 Chemistry Chapter 10 The S-Block Elements
- NCERT solutions for Class 11 Chemistry Chapter 10 The S-Block Elements
- NCERT Exemplar Class 11 Chemistry Solutions Chapter The S-Block Elements
Structure of Potassium Hydroxide
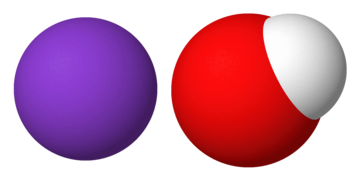
(3 D structure of KOH)
The chemical formula for potassium hydroxide is KOH. Hydroxide (OH–) is made up of one hydrogen and one oxygen atom with a charge of -1. The alkali metal potassium (K+) has a charge of +1. As a result, the equation is balanced, and the charges are distributed evenly. The chemical formula of caustic potash is KOH. The distance between K+ and OH- ions lies between 2.69 and 3.15 A◦.
Although KOH can exist as a crystalline solid comparable to sodium chloride, it usually forms crystalline hydrates due to its great affinity for water. The most frequent hydrates of caustic potash are mono, di, and tetra, which have one, two, or four water molecules bonded to one molecule of potassium hydroxide, respectively.
Solubility of Potassium Hydroxide
Potassium hydroxide is highly soluble in water, ethanol, methanol, and glycerin.
Potassium hydroxide is mildly soluble in ether.
Preparation of Potassium Hydroxide
Electrolysis of KCl
Electrolysis of potassium chloride solution produces potassium hydroxide on an industrial scale. The process is also known as the chloralkali process.
2KCl + 2H2O → KOH + Cl2 + H2
Salt metathesis Reaction
Though KOH is now made by electrolysis with KCl, it was previously made by a salt metathesis reaction of calcium hydroxide and potassium carbonate. The two reactant compounds react to generate two new compounds as products in a salt metathesis process, where their respective components or constituents change places.
Ca (OH) 2 + K2CO3 → CaCO3 + KOH
Related Topics Link |
Potassium Hydroxide Uses:
When compared to NaOH, potassium hydroxide solution is more conductive, hence it is utilised as an electrolyte in some alkaline batteries.
In the food sector, it is utilised as a pH control agent.
It is a thickening agent for food.
It is employed in semiconductor chip production.
It is used to make cuticle removers, which are used for manicure treatments.
It aids in the identification of fungus species.
Cotton is mercerized with it.
In analytical chemistry, it is employed in alkalimetric titrations.
It is used in the production of liquid fertilisers.
NCERT Chemistry Notes:
Chemical Properties of Potassium Hydroxide
Saponification of ester
The ester is saponified in a sealed tube by heating it with a specified amount of potassium hydroxide in an organic solvent. This reaction must be quantifiable and take an acceptable amount of time to be useful analytically. The use of a strong base is one circumstance that promotes a quick and quantitative reaction.
KOH + RCOOR1 → RCOOK + R1OH
Reaction with Carbon Dioxide
When potassium hydroxide comes into contact with CO2, it produces bicarbonate.
Because the hydroxide ion combines with carbon dioxide to generate bicarbonate alkalinity, adding hydroxide ions such as lime, sodium hydroxide, or potassium hydroxide modifies the pH.
KOH + CO2 → KHCO3
Reaction with zinc
With the chemical formula KOH, potassium hydroxide is a strong inorganic alkaline substance. It is very corrosive to metal and tissue since it is a noncombustible, hydrophilic material. It easily absorbs moisture from the air and produces a caustic solution that corrodes aluminium and zinc.
KOH + Zn → K2ZnO2 + H2
Health Hazards of Potassium Hydroxide
Potassium hydroxide's health risks are similar to those of other powerful alkalis, such as sodium hydroxide. Skin, mucous membranes, and eyes can be severely irritated by potash lye and its solution. It can generate heat and cause combustion when it comes into touch with water or moisture. Tissues are corroded by potassium hydroxide.
Also check-
Frequently Asked Questions (FAQs)
Potassium hydroxide, sometimes known as caustic potash, is utilised in numerous industries. The most common uses are potassium carbonate, potassium phosphates, liquid fertilisers, and potassium soaps and detergents.
KOH is an example of a strong base, meaning it dissociates into its ions in aqueous solution. Although KOH, or potassium hydroxide, has a very high pH (normal solutions vary from 10 to 13), the exact value is determined by the concentration of this strong base in water.
The inorganic compound potassium hydroxide, popularly known as lye, has the chemical formula KOH. It is also known as caustic potash, and it is a powerful base that comes in a variety of forms, including pellets, flakes, and powders. It has a wide range of applications in the chemical, mining, and manufacturing industries.
Eye pain, tears, redness, and swelling are all symptoms of this condition. Larger doses result in severe burns and the possibility of blindness. Chronic exposure: frequent exposure to dilute solutions or potassium hydroxide dust causes tissue destruction.
When potassium reacts with water, it produces potassium hydroxide and hydrogen gas. The resulting products will be colourless. This is an exothermic process in which the hydrogen emitted interacts aggressively with oxygen and ignites. Water-soluble potassium compounds are possible.
K(s) + H2O → KOH (aq) + 1/2 H2
Also Read
19 Feb'25 10:44 AM
17 Dec'24 10:24 AM
17 Dec'24 10:21 AM
29 Nov'24 12:13 PM
30 Sep'24 02:31 PM
30 Sep'24 11:46 AM
26 Sep'24 06:10 PM
26 Sep'24 05:53 PM

