Carbonic Acid - Structure, Importance, Properties & Uses, FAQs
Carbonic acid has a chemical formula H2CO3. There is a small amount of this compound in the solution of carbon dioxide in water. Since the compound contains one carbon-oxygen double bond, its chemical carbonic acid formula is (H2CO3). The carbon dioxide chemical formula is CO2. As the only acid exhaled in its gaseous state by the lungs, carbonic acid is often described as a respiratory acid. Carbonate and bicarbonate salts are formed by it; it is a weak acid.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Carbonic acid structure
- H2CO3 properties
- 1. Physical-chemical properties
- 2. Chemical Properties
- Carbonic Acid Uses
- Blood Carbonic Acid: Its Importance
- Importance of Carbonic Acid in Oceans
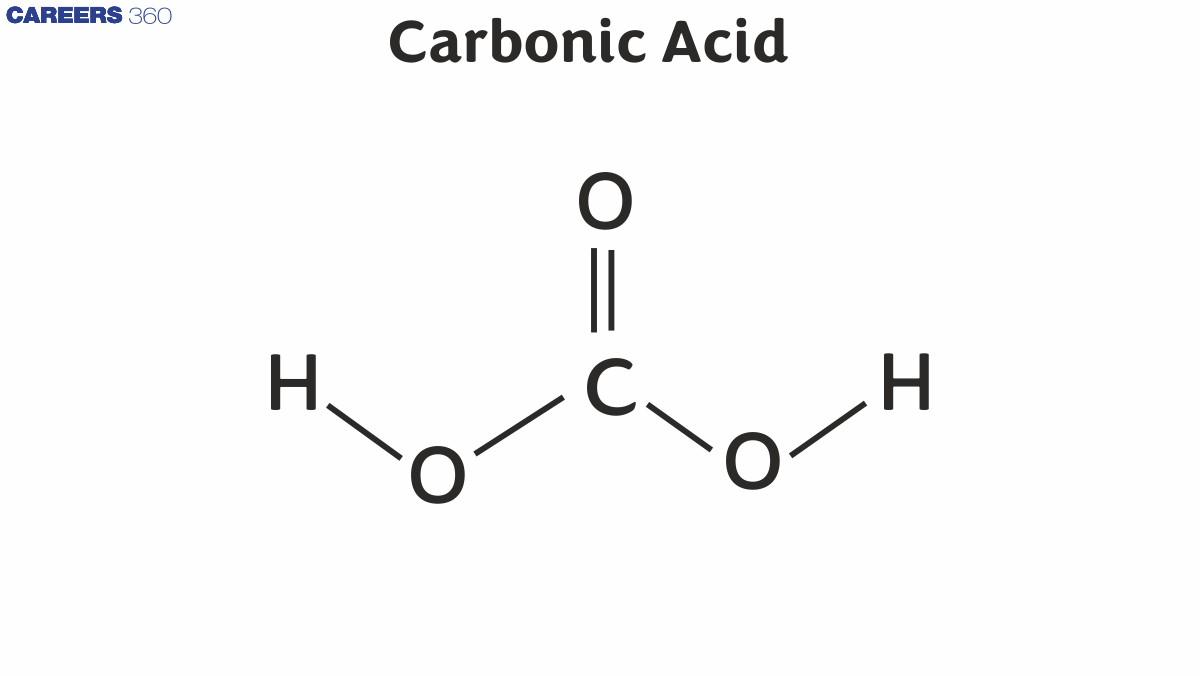
Carbonic acid structure
Below is an illustration of carbonic acid's structure.
As demonstrated by the illustration above, carbonic acid has a structure that includes a carbon-oxygen double bond and two carbon-oxygen single bonds. One Hydrogen atom is attached to each oxygen atom participating in a single bond with the carbon.
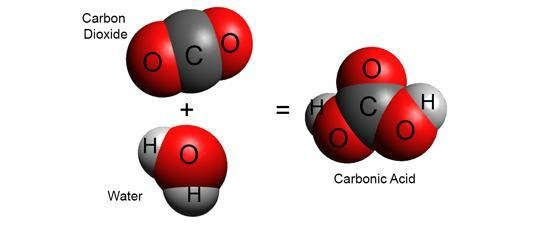
Carin water, and carbon dioxide participate in the following chemical equilibrium :
CO2 + H2O ⇌ H2CO3
Carbon dioxide in water is converted into carbonic acid in the equilibrium described above, but it is a very small fraction. Carbon dioxide (chemical carbonic acid formula CO2) is an acidic colorless gas with a density about 53% higher than that of dry air.
Also read :
H2CO3 properties
Under this subsection, you will find a list of some of the significant physical and chemical properties of carbonic acid.
1. Physical-chemical properties
- Carbonic acid has a molar mass of 62.024 grams per mole.
- 1.668 grams per cubic centimeter is its density in its standard state.
- In chemical terms, H2CO3 has a p Ka of 6.35.
- As a conjugate base, bicarbonate corresponds to carbonic acid.
- In most cases, this compound is dissolved in water. There have been reports that NASA scientists have prepared solid H2CO3 samples.
2. Chemical Properties
- A weak acid, H2CO3 is inherently unstable in nature.
- When water is present, it dissociates partially to give H+ and HCO3– (bicarbonate) ions.
- A diprotic acid such as carbonic acid can form two types of salts, both of which are bicarbonates.
- Bicarbonate salts are formed by adding a small quantity of a base to H2CO3, whereas carbonate salts are formed by adding an excess of a base.
- Interestingly, industrial fermentation processes or the burning of fossil fuels can result in the production of carbonic acid by-products.
Related Topics |
Carbonic Acid Uses
With a wide range of uses, H2CO3 is a very useful compound. Carbonic acid is also used in the following ways.
- It is carbonic acid that is used to prepare carbonated water, sparkling wine, and other aerated drinks.
- Ammonium persulfate is precipitated from H2CO3 powder.
- By transporting carbon dioxide from the body, it helps to eliminate it.
- Ringworm and other dermatitis are treated by protonating certain nitrogenous bases in blood serum and applying a solution of carbonic acid to the area.
- Cleansing contact lenses with these solutions is extremely effective.
- When necessary (such as during drug overdoses), it can be consumed orally to induce vomiting.
Blood Carbonic Acid: Its Importance
By facilitating the process of breathing gas exchange, the bicarbonate ion facilitates the transport of carbon dioxide out of the human body.
It is quite slow for carbon dioxide to undergo hydration reactions, especially without the assistance of a catalyst. the red blood cells contain the enzyme family known as carbonic anhydrases, which contributes to the higher reaction rate.
Catalyzing the dissociation of carbon dioxide and water into carbonic acid is the role of carbonic anhydrase Enzymes. Blood plasma is dissolved in bicarbonate anions created by this process. Upon exhalation, CO2 is formed from the catalyzed reaction in the lungs.
Importance of Carbonic Acid in Oceans
oceans are believed to have shifted the pH of the ocean water by approximately 0.1 due to the absorption of excess carbon dioxide from the atmosphere (primarily caused by human activities). Ocean water reacts with carbon dioxide to form hydrogen carbonate. Ocean acidification is a common term used to describe this process.
Also check-
| NCERT notes Class 12 Chemistry | NCERT Exemplar Class 12th Chemistry Solutions |
| NCERT Notes For All Subjects | NCERT Exemplar Solutions for All Subjects |
Frequently Asked Questions (FAQs)
Besides soft drinks, artificially carbonated sparkling wines, and other bubbly beverages, carbonic acid is also used to make artificially carbonated sparkling wine. Bicarbonates (or hydrogen carbonates) and carbonates are the salts of carbonic acid.
Carbonic acid is a hydroxide that contains substituted hydroxyl groups. Additionally, it is a polyprotic acid. Since this compound has two protons, it is diprotic and therefore belongs to a group of chemicals called diprotic compounds. The dynamics of dissociation can be described using two constants, where the first is for dissociation into the bicarbonate ion.
Carbon dioxide is expelled from the body by bicarbonate, a chemical intermediate. Generally, CO2 does not hydrate fast without a catalyst, but red blood cells release a substance known as carbonic anhydrase, which speeds up the metabolism of CO2, forming dissolved bicarbonate (HCO3– ) in the blood plasma.
A strong acid is a carbonic acid, not carbonic acid itself. Weak acids such as H2CO3 are dissociated into proton-containing cations (H+ cations) and bicarbonate-containing anion-forming cations (HCO3– cations). The compound does not completely dissociate in water. As a matter of fact, the bicarbonate ion of carbonic acid, which is the conjugate base of carbonic acid, is a relatively good base. Due to these reasons, carbonic acid is classified as a weak acid rather than as a strong acid.
Carbonic acid is formed when carbon dioxide gas dissolves in water. The reaction can be represented as:
[$ \text{CO}_2 + \text{H}_2\text{O} \rightleftharpoons \text{H}_2\text{CO}_3 $]
This equilibrium can shift based on factors like pressure and temperature.
Also Read
19 Dec'24 11:20 PM
17 Dec'24 10:22 AM
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM