Chemical Equation - Meaning, Definition, Examples, Representation, FAQs
A chemical equation is a symbolic representations of chemical reactions in which the reactants and products are expressed in terms of chemical formulae. Symbols are also utilised to represent the direction of the reaction as well as the actual states of the responding entities. Jean Beguin, a French chemist, was the first to formulate chemical equations in 1615. Chemical reactions can be expressed on paper with the use of chemical reaction formula.
This Story also Contains
- Represention of Direction of the Chemical Reaction
- Representing the Physical States of the Reacting Entities
- Representation of Energy in a Chemical Equation
- Ionic Chemical Equations
- Some Solved Examples
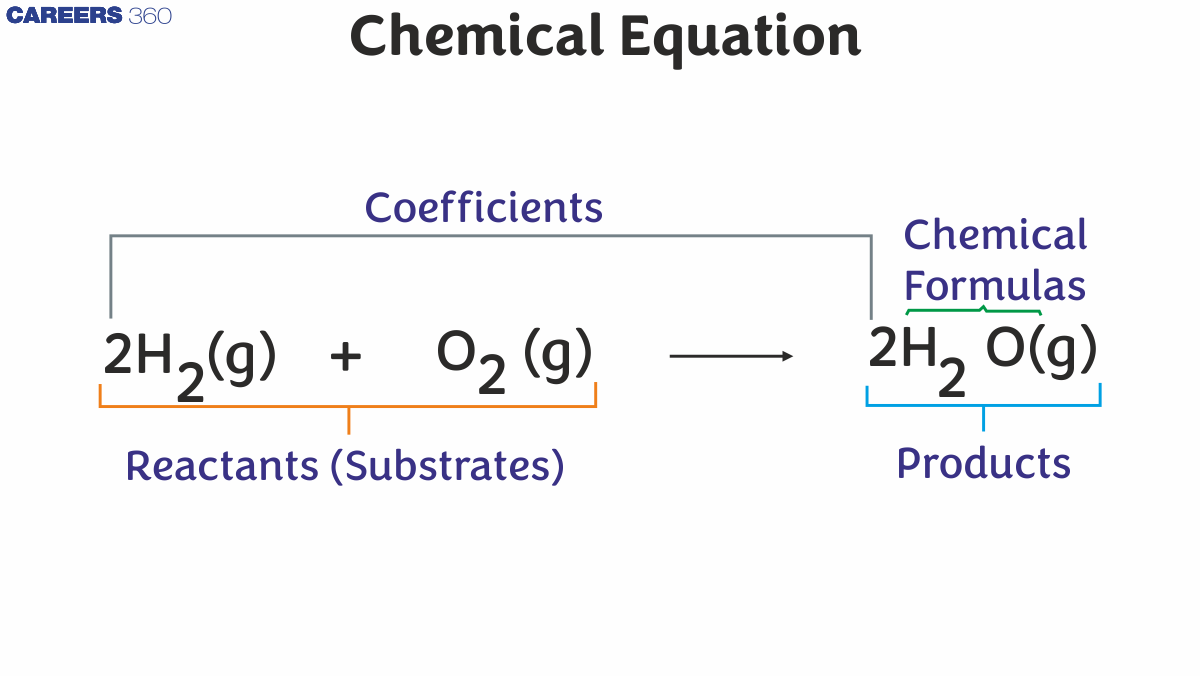
For example
$2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}$
The reactants are written on left hand side of chemical equation, while the products created by the chemical reactions are written on the right-hand side. It's also worth noting that each of the symbols for the respective reactants and products has a coefficient associated with it. The exact value of the stoichiometric number for each entity is represented by the coefficients of entities in a chemical equation.
Represention of Direction of the Chemical Reaction
One of the four symbols below can be used to separate the reactants and products (for which chemical formulae are stated in chemical equations).
• The symbol ‘→’ is used to represent a net forward reaction.
• The sign ‘⇌’ is used to represent a condition of chemical equilibrium.
• The ‘=' symbol is used to represent stoichiometric relationships.
Representing the Physical States of the Reacting Entities
In addition to the stoichiometric coefficients of the responding and generated entities, symbols enclosed in parenthesis are written next to them to describe their physical states during the chemical reaction. These symbols could be any of the ones listed below.
• The symbol (s) designates a solid-state entity.
• The symbol (l) indicates that an entity is in the liquid state; the symbol (g) indicates that the entity is in the gaseous state.
• In a chemical equation, the (aq) symbol for an entity denotes an aqueous solution of that entity.
A reactant or a product in some reactions may take the form of a precipitate that is insoluble in the solution in which the reaction is taking place. To describe these things as precipitates, the ‘↓’ symbol is put next to their chemical formula.
Also read -
Representation of Energy in a Chemical Equation
Some chemical processes necessitate the addition of energy to continue. The energy requirements of these reactions are represented by the symbols above the arrow symbol (forward reaction) in their respective chemical equations. The symbol aq represents the compound is aqueous.
The Greek letter delta (Δ) is used to indicate that the reaction requires a heat energy input, whereas the formula ‘hv', which specifies the energy of a photon, is used above the arrow sign to indicate that the reaction requires a light input to continue. It's worth noting that the stoichiometric coefficients provided to each entity in the chemical equation are employed to ensure that the entire equation follows the laws of charge and mass conservation.
|
Related Topics |
Ionic Chemical Equations
Electrolytes (substances that break down into ions when dissolved in polar solvents) are broken up and expressed as individual ions in ionic chemical equations. Single displacement reactions and salt metathesis processes can be described using these equations (generally referred to as double displacement reactions).
The following is an example of an ionic chemical equation.
Chemical equation: $\mathrm{CaCl}_2+2 \mathrm{AgNO}_3 \rightarrow \mathrm{Ca}\left(\mathrm{NO}_3\right)_2+2 \mathrm{AgCl} \downarrow$
Ionic Equation: $\mathrm{Ca}^{2+}+2 \mathrm{Cl}^{-}+2 \mathrm{Ag}^{+}+2 \mathrm{NO}_3^{-} \rightarrow \mathrm{Ca}^{2+}+2 \mathrm{NO}_3^{-}+2 \mathrm{AgCl}_{\downarrow}$
The Ca2+ (calcium ion) and the NO3– (nitrate) ions are present on both sides of the ionic equation when comparing the reactants and products of the ionic equation and the chemical equation. Because they do not participate in the chemical reaction, these ions are referred to as spectator ions.
By deleting the spectator ions and only stating the reaction between the participating ions, the net ionic equation for the case above can be written as shown below.
$2 \mathrm{Cl}^{-}+2 \mathrm{Ag}^{+} \rightarrow 2 \mathrm{AgCl} \downarrow$
This ionic chemical equation can be translated as follows: two chloride ions derived from calcium chloride react with two silver cations derived from silver nitrate, resulting in a silver chloride precipitate as a product.
Also check-
Some Solved Examples
Question 1: Which of the following is a balanced chemical equation?
A. $\mathrm{Ca}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2$
B. $2 \mathrm{Ca}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2$
C. $\mathrm{Ca}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2$
D. $\mathrm{Ca}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CaOH}+\mathrm{H}_2$
Solution:
Check atoms on both sides:
Ca = 1, H = 4, O = 2 → balanced in option C.
hence, the correct answer id option (C)
Question 2: In the reaction
$2 \mathrm{KClO}_3 \rightarrow 2 \mathrm{KCl}+3 \mathrm{O}_2$
How many moles of oxygen gas are produced from $\mathbf{1}$ mole of $\mathrm{KClO}_3$ ?
A. 1 mol
B. 1.5 mol
C. 2 mol
D. 3 mol
Solution:
$\begin{aligned} & 2 \mathrm{~mol} \mathrm{KClO}_3 \rightarrow 3 \mathrm{~mol} \mathrm{O}_2 \\ & 1 \mathrm{~mol} \mathrm{KClO}_3 \rightarrow \frac{3}{2}=1.5 \mathrm{~mol} \mathrm{O}_2\end{aligned}$
hence, the correct answer id option (B)
Question 3: Which law is obeyed by a balanced chemical equation?
A. Law of definite proportions
B. Law of multiple proportions
C. Law of conservation of mass
D. Law of reciprocal proportions
Solution:
Balancing ensures total mass of reactants = total mass of products.
hence, the correct answer id option (C)
Question 4: The skeletal equation for the combustion of ethane is:
$\mathrm{C}_2 \mathrm{H}_6+\mathrm{O}_2 \rightarrow \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
The balanced equation is:
A. $2 \mathrm{C}_2 \mathrm{H}_6+5 \mathrm{O}_2 \rightarrow 4 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O}$
B. $2 \mathrm{C}_2 \mathrm{H}_6+7 \mathrm{O}_2 \rightarrow 4 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O}$
C. $\mathrm{C}_2 \mathrm{H}_6+3 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2+3 \mathrm{H}_2 \mathrm{O}$
D. $\mathrm{C}_2 \mathrm{H}_6+4 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2+3 \mathrm{H}_2 \mathrm{O}$
Solution:
Balance C → H → O systematically. Option B satisfies all atoms.
hence, the correct answer id option (B)
Frequently Asked Questions (FAQs)
Chemical formulae and symbols are used to represent chemical reactions in these equations. The reactants are on left-hand side of chemical equation, as well as products are on right-hand side.
Ionic equations are chemical equations in which electrolytes are represented as dissociated ions.
Below are some of the examples of the chemical equations:
PCl5 + 4H2O → H3PO4 + 5HCl
SnO2 + 2H2 → 2H2O + Sn
TiCl4 + 2H2O → TiO2 + 4HCl
H3PO4 + 3KOH → K3PO4 + 3H2O
Na2S + 2AgI → 2NaI + Ag2S
The following symbols are used to represent the physical states of the reacting entities:
(s) stands for solid.
(l) stands for liquid.
(g) is the unit of measurement for gas.
The symbol for a chemical's aqueous solution is frequently used (aq).
Coefficients indicate the moles or relative amounts of each substance involved in the reaction, ensuring the equation is balanced.
