Elements and Compounds - Definition, Example, Types, Classification, FAQs
Have you ever wondered what the entire universe is made of? How do we see an endless variety of substances around us? What did they make of? What is it that makes oxygen different from hydrogen, or iron different from copper? You will get these answers by reading this article on elements and compounds. An element is a pure substance that cannot be broken down into simpler substances by chemical means. It is made up of only one type of atom.
This Story also Contains
- Classification of Matter
- Element
- Examples of Elements
- Types of Elements
- Compound
- Examples of Compounds
- Types of compounds
- Some Solved Examples
.jpg)
A compound is a pure substance that is formed when two or more different elements chemically combine in a fixed proportion. Unlike elements, a compound can be broken down into simpler substances by chemical methods
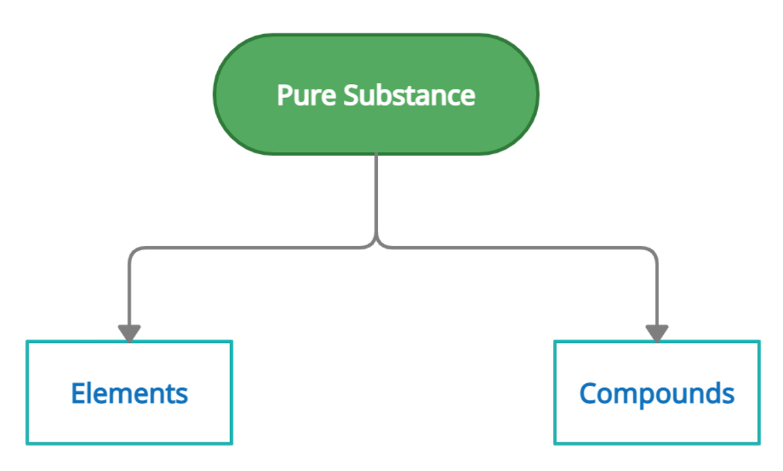
Classification of Matter
Chemistry is the study of different types and properties of matter. We see different types of components and materials all around us. It is therefore essential to classify and categorize them so that they can study, analyze and understand their characteristics easily of elements compounds, and mixtures. The matter around us can be classified as pure matter and impure matter. Those with fixed chemical composition are pure substances. A pure substance is divided into elements and compounds. Since impure substances are substances that may have different compositions and therefore do not have any fixed characteristics thus, they can't be divided into elements and compounds.
Element
A substance that cannot be broken down into simpler components using chemical methods or A substance composed of atoms with one atomic number is called an Element. An element meaning is a complete chemical substance that constitutes an entry in the modern periodic table. Elements and compounds are made up of only one type of atom and two or more elements and compounds respectively. They cannot be broken down into simpler fragments and can exist in the form of atoms or molecules. The elements are represented by symbols, issued by IUPAC. For example, Hydrogen is represented by H, Nitrogen is represented by N, and so on.
Examples of Elements
Below we have listed 10 examples of elements,
|
Element |
Symbol |
|
Hydrogen |
H |
|
Helium |
He |
|
Lithium |
Li |
|
Beryllium |
Be |
|
Boron |
B |
|
Carbon |
C |
|
Nitrogen |
N |
|
Oxygen |
O |
|
Fluorine |
F |
|
Neon |
Ne |
Related Topics Link
Types of Elements
Elements are classified into metals, non-metals, and semi-metals according to their properties.
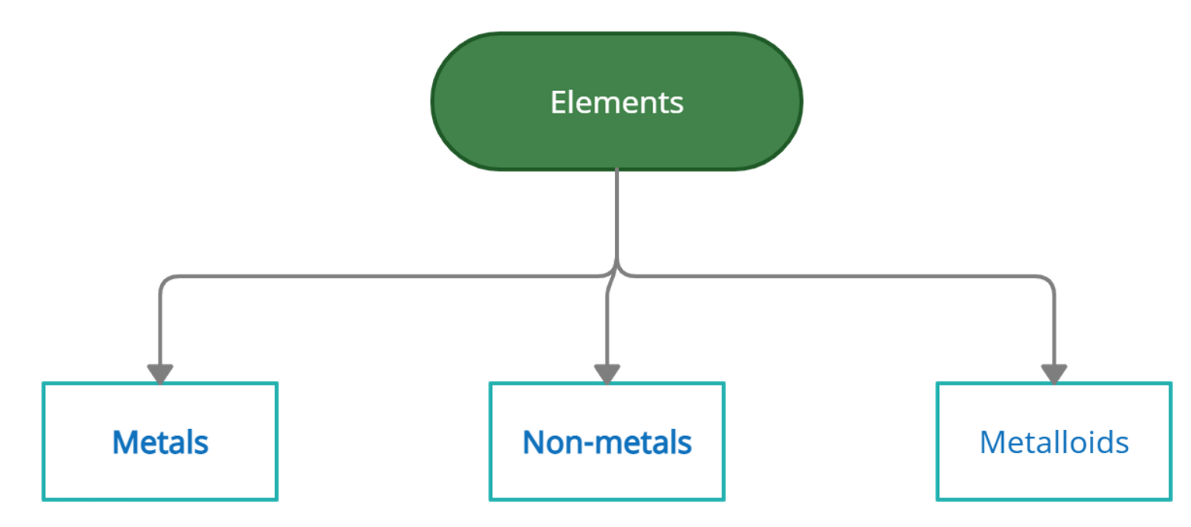
Metals: Metals are the electro positivity elements that tend to lose electrons in order to achieve stability. Metals have physical characteristics such as hardness, high stresses, lusters, conductivity, high melting and boiling points, etc.
Examples: Iron (Fe), Sodium (Na), Calcium (Ca), Lead (Pb), etc.
Non-metals: Non-metals are the elements that tend to get electrons, i.e. they are electron-negative. They are not metals. Non-metallic physical properties include fragility, comparatively less strength of the tensile materials, brightness, non-conductivity or isolation, lower melting and boiling points, etc.
Example: Hydrogen (H), Chlorine (Cl), Iodine (I), Phosphorus (P), etc.
Metalloids: Metalloids are elements with properties between metals and nonmetals.
Example: Boron (B), Germanium (Ge), Silicon (Si), etc.
Read more :
- NCERT notes Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
- NCERT solutions for Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 1 Some basic concepts of Chemistry
Compound
A compound is a pure substance formed when two or more different elements chemically combine in a fixed ratio by mass. The elements in a compound are held together by chemical bonds (ionic or covalent), and the compound has properties different from its constituent elements.
Key Points
- Composition is fixed (definite proportion).
- Components cannot be separated by physical methods (need chemical methods).
- Has new physical and chemical properties.
Examples of Compounds
Below we have listed 10 examples of compounds,
|
Compound |
Formula |
|
Water |
H2O |
|
Sulphuric Acid |
H2SO4 |
|
Alcohol | $\mathrm{C}_2 \mathrm{H}_4 \mathrm{O}$ |
|
Common Salt |
NaCl |
|
Nitrous oxide |
N2O |
|
Carbon Dioxide |
CO2 |
|
Glucose | $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6$ |
|
Ammonia |
NH3 |
|
Acetic Acid | $\mathrm{C}_2 \mathrm{H}_4 \mathrm{O}_2$ |
|
Copper sulphate |
CuSO4 |
Types of compounds
Compounds can be categorized as ionic and covalent compounds on the basis of formation.
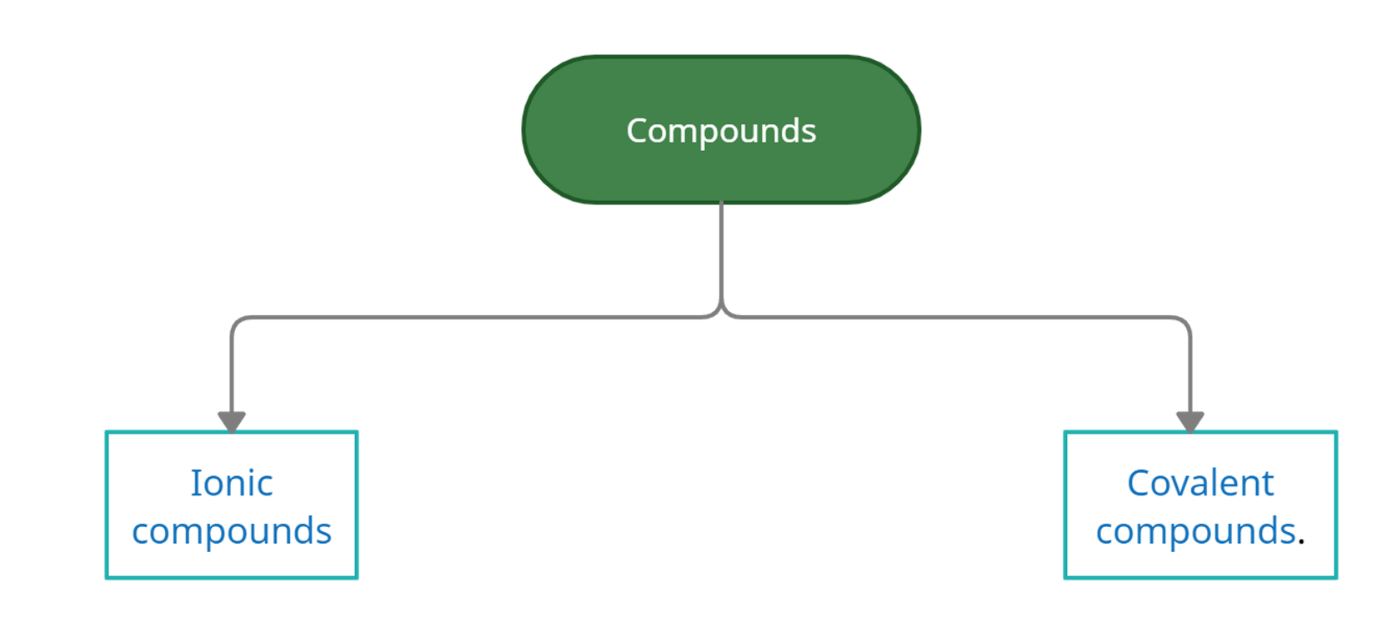
Ionic Compound: Between metal and non-metal, ionic compounds are formed. They are also known as electrovalent ion compounds. The metal atom is cation-forming and the non-metal atom is forming an anion. Electrons are anions.
Examples: sodium chloride, calcium oxide, etc.
Covalent compound: Between two non-metals covalent compounds are formed. It is also known as a molecular compound. The electrons are formed by the sharing of two or more nonmetals.
Example: water, carbon dioxide, methane, sugar, etc.
Also read -
Some Solved Examples
Question 1: Which of the following is known as dry ice?
1) Gaseous CO2
2) Solid SiO2
3) CH4
4) (correct) Solid CO2
Solution:
Solid CO2 is called as dry ice.
Hence, the answer is option (4).
Question 2: Which of the following is an element?
A) $\mathrm{H}_2 \mathrm{O}$
B) NaCl
C) $\mathrm{O}_2$
D) $\mathrm{CO}_2$
Solution:
An element consists of only one type of atom. O₂ contains only oxygen atoms, so it is an element. The others are compounds made of more than one type of element.
Hence, the answer is option (3).
Question 3: Which of the following statements is true for a compound?
A) It can be separated into simpler substances by physical methods.
B) It has properties identical to its constituent elements.
C) It is formed by the chemical combination of elements in a fixed ratio.
D) It contains only one type of atom.
Solution: Compounds are chemically combined substances with fixed composition. Their properties are different from the elements that form them, and they cannot be separated by physical methods.
Hence, the answer is option (3).
Question 4: Which of the following is a compound containing both metallic and non-metallic elements?
A) $\mathrm{O}_2$
B) NaCl
C) $\mathrm{N}_2$
D) $\mathrm{H}_2$
Solution: NaCl is formed from sodium (metal) and chlorine (non-metal). O₂ and N₂ are elements, and H₂ contains only hydrogen.
Hence, the answer is option (2).
Question 5 : Which statement is true for a compound?
A. Components can be separated physically
B. Composition is variable
C. Properties are same as constituents
D. Definite chemical formula exists
Solution:
A compound is formed when two or more elements chemically combine in a fixed ratio. Therefore, every compound has a definite chemical formula (for example, $\mathrm{H}_2 \mathrm{O}, \mathrm{NaCl}$).
Hence, the answer is option (4).
Frequently Asked Questions (FAQs)
Mixtures are structures that are physically combined and can be separated into original components. One type of atom or molecule is a chemical substance for elements and compounds. A combination is made up of various kinds of atoms or molecules not chemically linked.
Example: A mixture of Sand and water, salt and water, etc.
Compound meaning is that which always contains the same element definition in chemistry in the same proportions; for example, each carbon atom in carbon dioxide always has two oxygen atoms, and each oxygen atom in water always has two hydrogen atoms. The entire structure of a compound is always identical.
Element definition is a substance that is made up of only one atom form. A compound is a material consisting of more than one bonded atom. A mixture is a combination of two or more unbounded compounds or compounds, each of which retains its own properties.
Water is a compound because it contains more than one element that is hydrogen and oxygen combined in the ratio of 2:1.
Gold is an Element. Gold is Element 79 in the periodic table and the symbol of gold is Au. In fact, there are 118 elements and compounds in a periodic table.
Water: For drinking and as a solvent.
Table salt: A key part of our daily diet, meat, and fish preservatives.
Sugar: Used as a Sweetener, flavoring, and as a coloring agent.
Methane: Used as natural gas and as a fuel.
air
water
oxygen
iron
Ans. Option (b) is correct.
