Chemical Formula - Overview, Importance, Types, List, FAQs
Have you ever wondered how chemists represent a substance using just a few symbols and numbers? How can a simple combination of letters like H₂O or CO₂ tell us about the types of elements present and their exact proportions in a compound? You find these answers by reading this article on chemical formula. Chemical formulas allow you to express any chemical compound using the symbols for the components that make it up.
This Story also Contains
- Importance of Chemical formula
- Types of Chemical formula
- Empirical Formula
- Structural Formula
- Projection Formula
- How to Write a Chemical Formula
- Chemical formula of Organic Compounds
- Chemical formula table:
- Some Solved Examples

All chemical formulas reveal the components that make up a compound's molecules, as well as the proportions in which these elements' atoms mix to create those molecules. The molecular formula H2O, for example, indicates that two hydrogen atoms unite with one oxygen atom to produce one water molecule. Similarly, sulfuric acid has the chemical formula H2SO4, indicating that the proportions of H, S, and O in this molecule are 2:1:4. So, in chemistry, all chemical formula is a shorthand representation of the components of a molecule, using the unique symbols given to each element in the periodic table.
Importance of Chemical formula
-
Chemical formulas give information about a compound's chemical composition.
-
They also show how the individual elements mix to produce the compound.
-
Chemical formula is crucial for students and professionals to predict, explain, and analyze chemical phenomena.
-
Ions, free radicals, and other chemical species can also be represented using chemical formulas.
- Aid in producing chemicals, pharmaceuticals, and other products by specifying the exact formulation required.
- Provide insights into the molecular structure and properties of substances.
Types of Chemical formula
Any of numerous types of expressions of the composition or structure of chemical compounds is referred to as a science chemical formula. The term "chemical formula" usually refers to a compound's molecular formula (which denotes the total number of atoms of each constituent element in one molecule of the compound). Empirical, molecular, structural, and projection formulae are the other types.
-
Molecular Formula: The number of components in a molecule may be determined using the molecular formula. The elements are designated by their respective symbols (as in the periodic table) in molecular formulas, and the number of atoms of each element in the molecule is written in subscript. The chemical formula for glucose, for example, is C6H12O6.
-
Empirical Formula: The ratio of the elements contained in a chemical compound is represented by the empirical formula. The majority of empirical formulas are derived through the examination of experimental data. CH2O is the empirical formula for glucose. The molecular formulas can be used to create empirical formulae.
-
Structural Formula: The structural formula of a chemical compound, as the name implies, reveals the atoms' arrangement in the molecule.
Empirical Formula
Ordinarily, empirical formulae are used to describe compounds with unknown molecular structures or chemicals that are not made up of common molecular entities, such as sodium chloride (table salt), which is made up of ions. A molecular formula is used to indicate the chemical makeup of a substance's constituent molecules (the molecule being the smallest unit in which the substance keeps its chemical characteristics).
As a result, benzene is represented by the empirical formula CH, indicating that a typical sample includes one atom of carbon (C) and one atom of hydrogen (H) (H). Water is represented by the empirical formula H2O, which indicates that it contains two hydrogen (H2) atoms for every atom of oxygen (O)
The chemical formula C2H4 and C3H6 for ethylene and propylene, for example, specify the number and type of atoms contained in each substance's molecule. Ethylene and propylene, on the other hand, have the same empirical formula, CH2, since they are both made up of carbon and hydrogen atoms in a 1:2 ratio. The empirical and molecular formulae of a material may be similar in some situations, such as water.
Structural Formula
Chemical bonds between atoms in a molecule are identified by structural formulae. A structural formula is made up of atom symbols linked by short lines that indicate chemical bonds—single, double, or triple bonds are represented by one, two, or three lines, respectively. The structural formula of ethane, for example, is
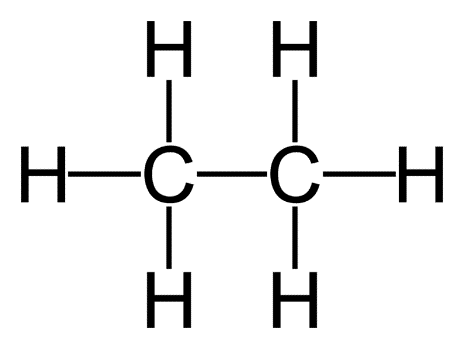
indicating that the molecule is made up of two carbon atoms, each connected to the other by a single bond, and three hydrogen atoms. Structural formulae are especially effective for demonstrating the differences between compounds of the same type and number of atoms.
Projection Formula
A projection formula is a two-dimensional depiction of a three-dimensional molecule that is truly two-dimensional. This sort of formula is similar to the structural type in that it is made up of symbols that represent atoms of the component elements linked by dashes or curves that represent chemical bonds. Thus, the projection formula neatly represents the methane molecule, which is defined by a tetrahedral arrangement of four chemical bonds around a carbon atom.
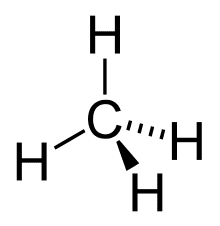
Stereoisomers are substances that have the same composition but differ in the spatial arrangement of the atoms that make up their molecules. Projection formulae are frequently employed in the study of stereoisomers. Stereoisomers can be recognised from one another by suitable variations in their formulae, thanks to certain standards for drafting projection formulas.
Also read :
How to Write a Chemical Formula
To create a chemical formula, we need to know the symbol for the elements in the compound, the radicals formula, and the valency of the elements in the compound. When constructing a chemical formula, keep the following considerations in mind.
-
The majority of the compounds are binary, meaning they only include two components. There are other compounds containing more than two elements.
-
A cation is an atom with a positive charge, while an anion is an atom with a negative charge.
-
The metal is named first, followed by the non-metal, in a combination comprising both metal and non-metal. For instance, NaCl is made up of Na+ (metal ion) and Cl– (chloride ion) (non-metal ion)
-
Anions with a -1 negative charge are generally suffixed with –ide. F− - Floride, for example.
-
Anions with oxyanions (oxygen + another element) are generally suffixed with –ate. For instance, SO42- (Sulphate)
-
Bi- or hydrogen is used as a suffix when a polyatomic anion has H– ion. HCO3-Bicarbonate or hydrogen carbonate are two examples.
-
Following are some examples of polyatomic anions:
|
Polyatomic Anion |
Basic Chemistry Formula |
|
Phosphate |
PO43- |
|
Cyanide |
CN- |
|
Amide |
NH2- |
|
Nitrate |
NO3- |
Chemical formula of Organic Compounds
Organic compounds are made up of a mixture of carbon and hydrogen, as well as nitrogen and a few additional elements including phosphorus, sulphur, silicon, and the halogens. The majority of organic chemicals are biologically derived, since they are present in nature.
-
Hydrocarbon: Hydrocarbons are substances made up entirely of carbon and hydrogen. Alkanes are hydrocarbons that have just single bonds between them. Methane is the most basic example (shown below). Ethene (C2H4) is the most basic alkene, with a double bond between the two carbon atoms.
Eg: Methane, Ethane
-
Functional Group: Atoms linked to carbon chains or rings of organic compounds form functional groups. Within a functional group, compounds tend to have similar qualities and characteristics. Hydroxyl and carboxyl groups are two typical functional groups. Alcohols are hydroxyl groups that terminate in -OH. Compounds having -COOH carboxylic acids have carboxyl groups that terminate in -COOH. Prefixes are used by functional groups to assist name compounds with comparable chemical characteristics, which helps with nomenclature.
Eg: Hydroxyl group. Carboxyl group
Chemical formula table:
Some of the chemical formula example listed below as compound names and formulas.
-
Common Acids
|
Name |
Chemistry Chemical formula |
|
Acetic acid |
CH3COOH |
|
Nitric Acid |
HNO3 |
|
Sulfuric Acid | $\mathrm{H}_2 \mathrm{SO}_4$ |
|
Hydrochloric Acid |
HCl |
|
Nitrous Acid |
HNO2 |
|
Sulphurous Acid | $\mathrm{H}_2 \mathrm{SO}_3$ |
|
Hyposulphurous Acid | $\mathrm{H}_2 \mathrm{SO}_2$ |
|
Carbonic Acid |
H2CO3 |
|
Phosphorous Acid |
H3PO4 |
|
Phosphoric Acid |
H3PO4 |
|
Oxalic Acid |
H2C2O4 |
|
Chromic Acid |
H2CrO4 |
|
Citric Acid |
C6H8O7 |
|
Formic Acid |
HCOOH |
2. Common Bases Chemical formula
|
Name |
Chemical formula |
|
Potassium Hydroxide |
KOH |
|
Lithium Hydroxide |
LiOH |
|
Calcium Hydroxide |
Ca(OH)2 |
|
Sodium Hydroxide |
NaOH |
|
Barium Hydroxide |
Ba(OH)2 |
|
Arsenic Hydroxide |
As(OH)3 |
|
Aluminium Hydroxide |
Al(OH)3 |
|
Ammonium Hydroxide |
NH4OH |
|
Mercury(I) Hydroxide |
Hg2(OH)2 |
|
Mercury(II) Hydroxide |
Hg(OH)2 |
|
Led (IV) Hydroxide |
Pb(OH)4 |
|
Tin (II) Hydroxide |
Sn(OH)2 |
-
Common Cations & Anions
|
Cations |
Formula |
Anions |
Formula |
|
Ammonium |
NH4+ |
Acetate |
CH3COO- |
|
Calcium |
Ca2+ |
Chloride |
Cl- |
|
Copper |
Cu2+ |
Fluride |
F- |
|
Potassium |
K+ |
Nitrite |
NO2- |
|
Iron |
Fe2+, Fe3+ |
Nitrate |
NO3- |
|
Sodium |
Na+ |
Oxide |
O2- |
|
Led |
Pb2+ |
Sulfate |
SO42- |
Most Important Ones
|
Chemical Compound |
Chemical formula of Compounds |
|
Aluminium Acetate |
C6H9AlO6 |
|
Aluminium Bromide |
AlBr3 |
|
Aluminium Carbonate |
Al(CO3)3 |
|
Aluminium Iodide |
AlI3 |
|
Aluminium Oxide |
Al2O3 |
|
Aluminium |
Al |
|
Ammonia |
NH3 |
|
Ammonium dichromate | $\mathrm{Cr}_2 \mathrm{H}_8 \mathrm{~N}_2 \mathrm{O}_7$ |
|
Ammonium bicarbonate |
NH4HCO3 |
|
Ammonium acetate |
C2H3O2NH4 |
|
Ammonium carbonate |
(NH4)2CO3 |
|
Ammonium nitrate |
NH4NO3 |
|
Ammonium oxide |
(NH4)2O |
|
Benzene |
C6H6 |
|
Benzoic Acid |
C7H6O2 |
|
Potassium bromate |
KBrO3 |
|
Butane |
C4H10 |
|
Calcium Acetate |
C4H6CaO4 |
|
Calcium Hydroxide |
Ca(OH)2 |
|
Calcium nitrate |
Ca(NO3)2 |
|
Carbon Tetrachloride |
CCl4 |
|
Chromic Acid |
H2CrO4 |
|
Iron oxide |
Fe2O3 |
|
Carbon monoxide |
CO |
|
Glycerol |
C3H8O3 |
The above table shows chemical formulas List.
Also read -
Some Solved Examples
Question 1: An organic compound contains $\mathbf{4 0} \boldsymbol{\%} \mathbf{C} \boldsymbol{,} \mathbf{6 . 6 7} \boldsymbol{\%} \mathbf{H}$, and $\mathbf{5 3 . 3 3} \boldsymbol{\%}$ O. What is its empirical formula?
A. $\mathrm{CH}_2 \mathrm{O}$
B. $\mathrm{C}_2 \mathrm{H}_4 \mathrm{O}_2$
C. CHO
D. $\mathrm{C}_3 \mathrm{H}_6 \mathrm{O}_3$
Solution:
Assume 100 g compound:
$\begin{aligned}
& \mathrm{C}=40 \mathrm{~g} \rightarrow \frac{40}{12}=3.33 \\
& \mathrm{H}=6.67 \mathrm{~g} \rightarrow \frac{6.67}{1}=6.67 \\
& \mathrm{O}=53.33 \mathrm{~g} \rightarrow \frac{53.33}{16}=3.33
\end{aligned}$
Divide by smallest (3.33):
$C: H: O=1: 2: 1$
Empirical formula $=\mathrm{CH}_2 \mathrm{O}$
Hence, the correct answer is option (A)
Question 2: The molecular mass of a compound is 180 u and its empirical formula $\mathrm{CH}_2 \mathrm{O}$. What is its molecular formula?
A. $\mathrm{C}_3 \mathrm{H}_6 \mathrm{O}_3$
B. $\mathrm{C}_4 \mathrm{H}_8 \mathrm{O}_4$
C. $\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6$
D. $\mathrm{C}_5 \mathrm{H}_{10} \mathrm{O}_5$
Solution:
Empirical formula mass $=12+2+16=30 \mathbf{u}$
$n=\frac{180}{30}=6$
Molecular formula $=\left(\mathrm{CH}_2 \mathrm{O}\right)_6=\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6$
Hence, the correct answer is option (C)
Question 3: What is the percentage of nitrogen in urea $\left(\mathrm{NH}_2\right)_2 \mathrm{CO}$ ?
A. 23.33%
B. 28.00 %
C. 46.67 %
D. 56.00 %
Solution:
Molar mass of urea $=60 \mathrm{~g}$
Mass of N = $2 \times 14=28 \mathrm{~g}$
$\text { Percentage }=\frac{28}{60} \times 100=46.67 \%$
Hence, the correct answer is option (C)
Question 4: Which of the following represents the correct chemical formula of washing soda?
A. $\mathrm{Na}_2 \mathrm{CO}_3$
B. $\mathrm{Na}_2 \mathrm{CO}_3 \cdot 7 \mathrm{H}_2 \mathrm{O}$
C. $\mathrm{Na}_2 \mathrm{CO}_3 \cdot 10 \mathrm{H}_2 \mathrm{O}$
D. $\mathrm{NaHCO}_3$
Solution:
Washing soda = Sodium carbonate decahydrate
Hence, the correct answer is option (C)
Frequently Asked Questions (FAQs)
An empirical formula shows the simplest whole-number ratio of elements, while a molecular formula indicates the actual number of atoms in a molecule.
Iron – Fe, Chlorine – Cl
H2O
Chemical formulas are important to understanding the composition, structure, and reactions of substances. They simplify complex chemical concepts and aid in scientific communication.
