Chemical Kinetics - Notes, Topics, Formula, Books, FAQs
Chemical kinetics is the branch of chemistry that deals majorly with the rates of chemical reactions. There are many reactions that occur instantaneously like AgNO3 with HCl to form a salt i.e., AgCl while there are many reactions that occur too slowly like the conversion of diamond into graphite. Along with feasibility, there are various other factors like rate and other parameters that control the rate of reaction. In this chapter, you will learn about the rates and order of the reactions, Arrhenius Equation , the Collision theory of reactions, etc.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Important Topics Of Chemical Kinetics
- Rate Of Chemical Reaction:
- Overview of the Chemical Kinetics
- How to prepare for Chemical Kinetics?
- Prescribed Books For Chemical Kinetics
Important Topics Of Chemical Kinetics
Rate Of Chemical Reaction:
The rate at which products are formed in the course of a reaction is called the Rate Of Reaction. It is a universal fact that there exists a wide extent of variation in rates in all chemical reactions. Of the chemical reactions, some take place almost instantaneously, while others, in general, take some time to reach the final equilibrium.
Elementary And Complex Reactions:
An elementary reaction is a one-step process where reactants directly go to form products. However, complex reactions have a number of steps and intermediates.
Order Of Reaction:
The order of reaction quantifies how the concentration of reactants affects the speed of a chemical reaction. In simpler terms, it is the sum of the powers to which the concentration terms are raised in the rate law expression.
Zero-Order Reactions:
Zero-order reactions are especially important in catalysis when the reaction rate is limited by the number of active sites on a catalyst, and not by the concentration of reactants. Rate of reaction of Zero Order Reaction is independent of concentration of the reactants.
First-Order Reactions:
First Order Reaction describe a scenario where the rate of reaction is directly proportional to the concentration of only one reactant. The rate of the reaction is proportional to the first power of the concentration of the reactant
Second-Order Reactions:
The case in which the rate is proportional to the product of two concentrations of two reactants or to the square of the concentration of a single reactant is called a Second Order Reaction.
Pseudo-First-Order Reactions:
A Pseudo First Order Reaction is a complex process involving more than one reactant or including complicated steps; however, it apparently obeys the first-order kinetics. Under certain conditions of the experiments, it simplifies to a first-order rate law, which usually happens when the concentration of one reactant is in large excess or if reaction intermediates are involved.
Arrhenius Equation:
The Arrhenius equation is a chemical kinetics equation that describes how the rate of a chemical reaction depends on temperature. It is also used to understand how increasing the temperature or decreasing the activation energy increases the rate of a reaction.
Molecularity Of Reaction:
Molecularity is referred to as the number of reactant molecules coming together simultaneously to collide with each other and enter into the transition state that finally leads to a chemical reaction. This approach is used to describe theoretically the mechanism of elementary reactions.
Rate Law:
The rate law of a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it.
Nth Order Reaction:
The nth-order reaction is one in which the rate of the reaction depends upon the concentration of one or more reactants raised to some power, which is called the order of the reaction. The order of a reaction may be an integer or even a fraction and is generally considered representative of how the rate of reaction depends on the concentration of reactants. Mathematically, this rate law may be defined for an nth-order reaction by the expression given below:
Rate=k[A]n
Overview of the Chemical Kinetics
(i) Rate of reaction: Similar to any object moving with a certain velocity, chemical reactions also occur with certain velocities also known as the rate of reaction. This rate of reaction is defined as how fast the product is forming or how fast the reactants are getting consumed with respect to time. Mathematically, it can be expressed as follows:
$\Delta [R] \,=\, [R]_{2}\, -\, [R]_{1}$
![\Delta [P] \, =\, [P]_{2}\, -\, [P]_{1}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/12/1649781777624.png) $\Delta t \, =\,t_{2}\, -\, t_{1}$
$\Delta t \, =\,t_{2}\, -\, t_{1}$
$\therefore\: Rate\, of\, reaction = \frac{Increase\, in\, concentration\, of\, P}{Time\, \, taken} = \frac{Decrease\, \, in\, concentration\,of\, R}{Time\,taken}$
$\therefore\: Rate\, of\, reaction = \frac{\Delta P}{\Delta t} = \frac{-\Delta R}{\Delta t}$
(ii) Rate expression and rate constant: For the general reaction:
$aA\, +\, bB\rightarrow cC\,+dD$
The rate equation is given as follows:
$Rate\,\alpha [A]^{x}\,[B]^{y}$
This equation can be written as follows:
$Rate\,=k[A]^{x}\,[B]^{y}$
where x and y may or may not be stoichiometric coefficients. The addition of these components (x+y) gives the order of the reaction.
(iii) Order of the reaction: The rate equation of a chemical reaction is given as:
$Rate\,=k[A]^{x}\,[B]^{y}$
where (x+y) gives the order of the reaction and k is the rate constant.
Units of rate constant: For a general reaction:
$aA\, +\, bB\rightarrow cC\,+dD$
$Rate\,=k[A]^{x}\,[B]^{y}$
$k\, =\, \frac{Rate}{[A]^{x}[B]^{y}}$
The unit of rate constant (k) depends on the order of the reaction i.e. (x+y).Reaction type
Order of reaction
Units of rate constant
Zero-order reaction
0
mol L-1 s-1
First order reaction
1
s1
Second order reaction
2
mol-1L s-1
Zero-order reaction: It is the type of reaction, where the order of reaction is proportional to the zero power of the concentration of reactants. The rate equation is given as follows:
$[R]\,=\,-kt\,+\,[R]_{0}$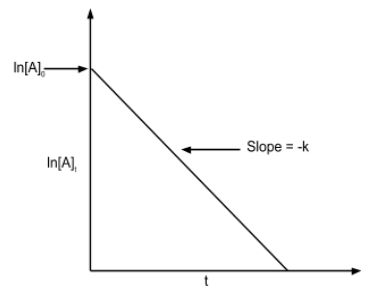
First order reaction: In these type of reactions, the rate of reaction is proportional to the first power of the concentration of reactants. The rate equation is given as follows:
$log\frac{[R]_{0}}{[R]}\,=\,\frac{k(t_{2}-t_{1})}{2.303}$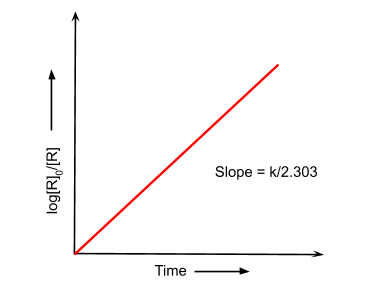
The half-life of reaction: It is the time at which the concentration of reactant is half of its initial value. It is denoted by t1/2. Mathematically, it can be described as follows:
$t_{1/2}\, =\, \frac{0.693}{k}$
Arrhenius Equation: The rate of a chemical reaction depends on the temperature. For every 100 rise in temperature, the rate constant gets doubled. This temperature dependency of the rate of chemical reaction is explained by the Arrhenius equation as given below:
k = A e-Ea/RT
where A is the Arrhenius factor, Ea is the activation energy and R is the gas constant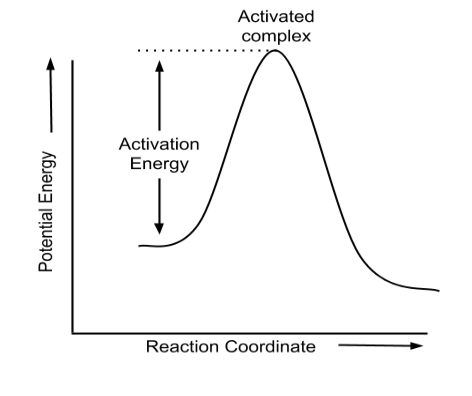
Collision Theory of Chemical Reactions: This theory gives a deeper insight into the mechanism of the chemical reactions. According to this theory, the reactant molecules are considered to be spherical molecules and the reaction occurs when these spherical molecules collide with each other. Mathematically, it can be described as follows:
Rate = ZAB eEa/RT
where ZAB is the collision frequency. The number of collisions per second per unit volume of the reaction mixture is known as "collision frequency(Z)".
How to prepare for Chemical Kinetics?
This chapter is a part of physical chemistry. There are some simple formulas and equations like zero order reaction, first-order reaction, etc. which you must understand completely because these concepts are the root of this chapter.
Before reading this chapter, first, you must have the basic knowledge of the mole concept.
You must deeply observe how the graphs show some trends, how to find the rate constants from the graphs, how to find the order of the reactions, etc.
Rest this chapter is very simple, just be regular and be consistent in your numerical practice.
NEET Highest Scoring Chapters & TopicsThis ebook serves as a valuable study guide for NEET exams, specifically designed to assist students in light of recent changes and the removal of certain topics from the NEET exam.Download EBook
In everyday life, there are various important chemical reactions which occur around us as follows:
Role of chemical kinetics in the "refrigerator": We keep our food items in the refrigerator because it lowers the temperature of these substances. As the temperature is lowered, the reaction rates are low and thus the food items can be used for longer times.

Role of chemical kinetics in the making of "popcorn": Each popcorn kernel contains a small amount of water in them. Now when the popcorn kernels are heated, then the water turns into the vapor which tries to come out of the kernel shell. Due to this heating and vapourization, the kernel shells are burst out with a 'pop' sound as the pressure is further increased.
In chemical kinetics, you also learn about how by changing the reaction conditions like temperature and pressure, the rate of reaction changes. It also gives you information about the reaction mechanisms, activation energy, and reaction intermediates..
Prescribed Books For Chemical Kinetics
First, you must finish the class XII NCERT book and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow R.C. Mukherjee and O.P. Tandon. You must definitely solve the previous year papers. Meanwhile, in the preparation, you must continuously write the mock tests for the depth of knowledge. This article will help you with a variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Frequently Asked Questions (FAQs)
Chemical kinetics is the study of the rates and mechanisms of chemical reactions. It involves the investigation of how fast or slow a reaction occurs and the factors that influence the reaction rate.
There are several factors that can affect the rate of a chemical reaction, including temperature, the concentration of reactants, the surface area of reactants, the presence of catalysts, and the nature of the reactants and products.
A reaction mechanism is a step-by-step process that explains how a chemical reaction occurs. It includes the intermediate species and the elementary reactions that lead to the formation of the final products.
A rate law is an equation that relates the rate of a chemical reaction to the concentration of the reactants. It provides information about the dependence of the reaction rate on the reactant concentrations and can be used to determine the reaction order and rate constant.
Chemical kinetics plays a crucial role in various fields, including chemical engineering, medicine, environmental science, and materials science. It can be used to design and optimize chemical reactions, develop new drugs and medicines, study atmospheric chemistry and pollution, and understand the behaviour of materials under different conditions.
Reversible reaction kinetics examines the rates at which chemical reactions occur in both forward and backward directions, considering factors such as concentration, temperature, and catalysts to describe the dynamic equilibrium between reactants and products
Also Read
19 Feb'25 04:56 PM
19 Feb'25 04:50 PM
19 Feb'25 04:42 PM
06 Feb'25 11:52 PM
20 Dec'24 01:21 PM
30 Sep'24 11:45 AM
25 Sep'24 09:40 AM
25 Sep'24 09:39 AM
24 Sep'24 09:46 PM
24 Sep'24 09:40 PM
