Chemistry in Everyday life - Notes, Topics, Formula, Books, FAQs
Chemistry is everywhere, whether it is the air we breathe, the food we eat, the medicines we use, the clothes we wear, or the occurrence of nuclear reactions. From the moment we wake up, we start using things associated with chemistry either directly or indirectly, like toothpaste that contains fluoride, the cleansing action of soap and detergent, the digestion of food, the conversion of food into energy, relieving pain from medicines, use of sodium benzoate and citric acid as food preservatives. In this chapter, students will learn about drugs, their classification, the action of drugs, artificial sweeteners, food preservatives and soaps, and detergents. There are various kinds of drugs that we use for therapeutic purposes, like Zantac, brompheniramine, etc. Chemistry is always at work.
This Story also Contains
- Important Topics- Chemistry in Everyday Life
- Overview of the Chapter
- Applications
- How to Prepare For This Chapter?
- Some Important PYQs
- Prescribed Books
Important Topics- Chemistry in Everyday Life
Classification Of Drugs:
Drug classification—this is the grouping of the arrangement of drugs according to established features: the structure, mechanism of action, therapeutic effect, and pharmacological features. One of the most broadly applied classifications, for example, is the Anatomical Therapeutic Chemical classification developed by the WHO. This class categorizes drugs based on their target organ or system and their therapeutic, pharmacological, and chemical properties.
Drugs And Drug Interactions:
Drug-target interactions refer to specific modes of action of drugs with biological molecules, mainly enzymes and receptors, in order to exhibit activities. One word: enzymes are proteins that catalyze biochemical reactions, so they are involved in drug metabolism. Being able to complex with an enzyme upon entering an organism, a drug can modify its activity.
Artificial Sweeteners And Sweetening Agents:
Artificial sweeteners and sweetening agents have really made a rapid rise in diets the world over. Artificial sweeteners are chemicals that produce a sweet taste like sugar but contain no calories. Sweeteners are formulated chemicals that are many times sweeter than sucrose. The most familiar ones are aspartame, saccharin, and sucralose.
Chemicals Used As Food Preservatives:
Chemical substances used to protect food materials against microorganisms are called preservatives. Food Preservatives are added to food products to help counter the action of bacteria, molds, fungi, and yeast. There are primarily two broad categories: natural and synthetic preservatives. Natural preservatives are substances derived from plants, animals, and microorganisms; examples include salt, vinegar, and some essential oils.
Tranquilizers - Antidepressant Drugs For Life:
Tranquilizers are a class of drugs that alleviate anxiety by relaxing an individual. They work mostly by producing sedation in the central nervous system. The commonly used tranquilizers are a group of benzodiazepines: diazepam, which is popularly referred to as Valium, and alprazolam, known in the market as Xanax.
Analgesics:
Analgesics are drugs used mainly to relieve pain. These drugs can, therefore, be classified into two major categories: non-opioid analgesics and opioid analgesics. Some examples of nonopioid medications that produce analgesia include acetaminophen, ibuprofen, and aspirin—over-the-counter drugs.
Antiseptics And Disinfectants:
Antiseptics reduce the number of or completely kill pathogenic microorganisms on living tissues, disinfectants do it on non-living surfaces or inanimate objects. Mechanisms of action in antiseptics and disinfectants are different.
Antibiotics:
Antibiotics represent very potent medicines designed to combat different kinds of bacterial infections. They act in two ways: bactericidal antibiotics kill bacteria directly, while the action of bacteriostatic antibiotics consists of slowing down the growth of bacteria until the immune system is able to fight off the infection.
Antifertility Drugs:
Antifertility drugs are artificially synthesized chemicals that aim at inhibiting the physiological processes of pregnancy. Normally, such drugs act by predominantly depressing the activity of hormones involved in ovulation and fertility. Synthetic derivatives of progesterone and estrogen form the most commonly used antifertility drugs, generally having a more significant effect than the natural hormones secreted by the body.
Soaps And Detergents:
Soaps and detergents are amphipathic molecules, meaning they have both hydrophilic and hydrophobic regions of the molecule. This unique characteristic gives them the capability of cleaning surfaces as well as lifting grease and dirt.
Overview of the Chapter
In this chapter, basically, you will study about three main topics in detail, i.e, drugs, food additives, and cleansing agents. Along with these insights, you will also get the proper tips and guidelines to prepare this chapter perfectly.
Drugs and their classification
Drugs are chemicals that have the property of interacting with the macromolecular targets and thus produce some biological response. Drugs are classified mainly into the following categories:
- On the basis of the pharmacological effect
- On the basis of drug action
- On the basis of chemical structure
- On the basis of molecular targets
Drug-Target interaction
In the body, proteins act as enzymes in biological reactions and also act as receptors in the body's communication system. In biological reactions, proteins as enzymes facilitate the reaction of the substrate with the reagent. When the substrate comes closer to the enzyme it binds to the enzyme and then with the help of a reagent, a chemical reaction happens and then the product is formed.
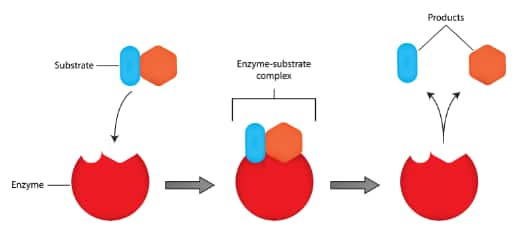
Now, drugs inhibit this activity of the enzyme. Drugs themselves bind to the active site of the enzyme and lock the space for the substrate to bind, and thus the chemical reaction does not proceed. This inhibition by drugs is done in two different ways:
(i) When the drug binds to the enzyme at the active site and thus no space is available for the substrate, and thus no chemical reaction proceeds.
(ii) When the drug binds to the enzyme at a different site, also known as an allosteric site, but due to this binding, the shape of the enzyme changes, and thus substrate is not able to bind with the enzyme, and thus no chemical reaction proceeds.
Therapeutic Action of Different Classes of Drugs
In this section, you will study the various kinds of drugs and their therapeutic effect.
- Antacids: Antacids are used for the treatment of acidity in the stomach. Traditionally, metal hydrogencarbonate and metal hydroxides were used for this treatment. Later on, a chemical was discovered known as histamine, which stimulated the secretion of pepsin and hydrochloric acid in the stomach. This was very helpful in the treatment of acidity and it was the largest-selling drug in the world until a new drug was discovered 'Zantac'.
- Antihistamines: Although histamine is used for the treatment of acidity but it has some allergic effects too, like it is responsible for widening of some vessels, nasal congestion, etc. Thurs, there are some synthetic drugs like brompheniramine and terfenadine which act as antihistamines. These drugs compete with histamines and reduce their activity.
- Neurologically Active Drugs: These are those drugs that affect the message transfer between the nerve to the receptor. These drugs are of two types viz:
(i) Tranquilizers
(ii) Analgesics - Antimicrobials: Antimicrobials are the chemicals that prevent or inhibit the action of various pathogens, such as bacteria, fungi, viruses, etc. There are various kinds of antimicrobials available, such as antibiotics, antiseptics, and disinfectants.
(i) Antibiotics
(ii) Antiseptics
(iii) Disinfectants - Antifertility Drugs: Antifertility drugs were developed for the control of the population. These drugs contain a mixture of synthetic estrogen and progesterone. Norethindrone and ethynylestradiol are the two most widely used antifertility drugs.
Chemicals in Food
There are some chemicals that are added to food for its preservation, color and nutritive value. Some of the food additives are as follows:
-
Artificial sweetening agents: Artificial sweeteners are used in place of traditional sweeteners to provide control to calories but still have a sweet taste. Traditional sweeteners like sucrose are sweet in taste but they add to the calorific value which is not recommended especially for diabetic patients. Some majorly used artificial sweeteners are aspartame, alitame, saccharin, sucralose, etc.
- Food preservatives: Food preservatives are those substances that are used for preserving food from any kind of spoilage. Some of the most commonly used food preservatives include sodium benzoate, sugar, table salt, salts of propanoic acid, etc.
Cleansing Agents
There are two types of detergents i.e, soaps and synthetic detergents which are used as cleansing agents. These cleansing agents are used to improve the cleansing properties of water.
-
Soaps: Soaps are basically the sodium and potassium salts of long-chain fatty acids. Soaps are formed by heating fat with aqueous sodium hydroxide solution, this process is known as saponification. Potassium soaps are softer to the skin than sodium soaps.
Soaps do not work in hard water because in hard water calcium and magnesium ions are present. These ions form insoluble scums in water and are useless for washing.
Types of Soaps: Basically, all soaps are made by following the same procedure as already described but they differ in the kind of raw material used in their formation.
- Synthetic Detergents: Synthetic detergents are powerful detergents and have all the properties of soaps. These detergents can be used in soft water as well as in hard water. These detergents can be classified into three categories:
(i) Anionic Detergents: These detergents are the sodium salts of sulfonated long-chain alcohols. These long-chain alcohols are treated with concentrated sulphuric acid to form alkyl hydrogen sulfates. Now, these alkyl hydrogen sulfates are neutralized by treating with alkali and thus form anionic detergents.
$\mathrm{CH_{3}(CH_{2})_{10}CH_{2}OH\, \overset{H_{2}SO_{4}}{\rightarrow}\, CH_{3}(CH_{2})_{10}CH_{2}OSO_{3}H\overset{NaOH(aq)}{\rightarrow}\, CH_{3}(CH_{2})_{10}CH_{2}OSO_{3}^{-}Na^{+}}$
(ii) Cationic Detergents: These detergents are the quaternary ammonium salts of amines. In the molecules of these detergents, the cationic part has a long chain of hydrocarbon and a positive charge on the nitrogen atom. One of the important cationic detergents is cetyltrimethylammonium bromide.
(iii) Non-ionic Detergents: These are detergents that do not contain any kind of ion on them. These detergents are formed by the reaction of stearic acid with polyethylene glycol.
$\mathrm {CH_{3}(CH_{2})_{16}COOH\, +\, HO(CH_{2}CH_{2}O)_{n}CH_{2}CH_{2}OH\, \overset{-H_{2}O}{\rightarrow}\, CH_{3}(CH_{2})_{16}COO(CH_{2}CH_{2}O)_{n}CH_{2}CH_{2}OH}$
These detergents remove grease and oil by micelle formation.
Applications
All these drugs, food additives, and cleansing agents that we study in this chapter have various real-life applications on a daily basis.
- For advanced diabetic patients, these people are not able to use the energy from the food and it was a very major problem. But insulin treatment solved this completely.
- For artificial sweeteners, aspartame is one of the most common in use. It is used to control the calorific value but with a sweet taste. In many soft drinks like Coca-Cola, aspartame is used as an artificial sweetener.

How to Prepare For This Chapter?
- This chapter is part of organic chemistry and possesses a very little weightage of marks in board exams and other competitive exams like JEE and NEET. It is completely theory-based. You are not supposed to memorize any formula.
- To prepare this chapter, you are not required to learn any prerequisite chapters.
- You are just supposed to memorize only some names of the medicines and some artificial sweetening agents because direct questions will be asked in the examination based on their names.
Some Important PYQs
Question: The $\mathrm{F}^{-}$ions make the enamel on teeth much harder by converting hydroxyapatite (the enamel on the surface of teeth) into much harder fluoroapatite having the formula.
(1) $\left[3\left(\mathrm{Ca}_3\left(\mathrm{PO}_4\right)_2\right) \cdot \mathrm{CaF}_2\right]$
(2) $\left[3\left(\mathrm{Ca}_2\left(\mathrm{PO}_4\right)_2\right) \cdot \mathrm{Ca}(\mathrm{OH})_2\right]$
(3) $\left[3\left(\mathrm{Ca}_3\left(\mathrm{PO}_4\right)_3\right) \cdot \mathrm{CaF}_2\right]$
(4) $\left[3\left(\mathrm{Ca}_3\left(\mathrm{PO}_4\right)_2\right) \cdot \mathrm{Ca}(\mathrm{OH})_2\right]$
Answer:
Fluoroapatite $\Rightarrow\left[3 \mathrm{Ca}_3\left(\mathrm{PO}_4\right)_2 \cdot \mathrm{CaF}_2\right]$
Hence, the answer is the option (1).
Question: Among the following, the number of tranquilizer/s is/are_____________
A. Chlordiazepoxide B. Veronal
C. Valium D. Salvarsan
(1) 3
(2) 2
(3) 4
(4) 1
Answer:
$\begin{array}{ll}\text { A. Chloroliazepoxide (Tranquilizer) } \\ \text { B. Veronal } & \text { (Tranquilizer) } \\ \text { C. Valium } & \text { (Tranquilizer) } \\ \text { D. Salvarsan } & \text { (Antibiotic) }\end{array}$
Hence, the answer is option (1).
Practice more questions from the link given below
For more questions to practice, the following MCQs will help in preparation of competitive examinations
Prescribed Books
For this chapter, first, the NCERT book is best for initial-level preparation as well as for board exams. Now, after this, if you want to prepare for competitive exams like JEE and NEET, then these are the best books for you - Morrison and Boyd and O.P Tandon. Meanwhile, in the preparation, you must continuously give mock tests to test the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Frequently Asked Questions (FAQs)
Chemistry is the branch of science that studies the properties, composition, and behavior of matter. It relates to everyday life in many ways, from the food we eat to the cleaning products we use. Chemical reactions occur everywhere around us when we cook, when we breathe, and even when we apply lotion to our skin.
Cleaning products contain various chemicals that are designed to break down dirt and stains. For instance, soaps and detergents contain surfactants that help lift dirt from surfaces. Chemistry helps us understand how these substances interact with oils and water, making cleaning more effective.
Chemistry is fundamental to biology, including our bodies' functions. Chemical reactions occur during digestion, metabolism, and even when our cells produce energy. Enzymes, which are proteins made of chemicals, facilitate these reactions, ensuring our bodies operate effectively.
pH measures how acidic or basic a substance is and plays a crucial role in various aspects of life. For example, our bodies maintain a specific pH level for optimal enzyme function. In gardening, pH affects nutrient availability in soil, making it essential for healthy plant growth.
Chemistry is key to food preservation methods such as canning, freezing, and drying. For instance, preservatives work by inhibiting the growth of bacteria, yeast, and molds. Understanding the chemical properties of these substances helps keep our food safe and fresh for longer periods.