Classification of Oxides - Dioxides, Example, Formula, Classification and FAQs
What are Oxides?
Oxide is an element that combines with the binary compound of oxygen to form a new element. Most metals in the periodic table react with oxygen to generate oxides. One element can often produce two or more oxides. The nature and properties of oxides vary greatly. When the elements react with oxygen, they produce dioxides (MO2) and tri oxides (MO ) (where M=Sulphur, Selenium, Tellurium, and other elements.
Example of Oxides
Carbon dioxide, sulphur dioxide, calcium oxide, carbon monoxide, zinc oxide, barium peroxide, and water are examples of oxides. Because oxygen is combined with only one element, these are called oxides.
Dioxides
Dioxide is an oxide that has two atoms of oxygen, each linked directly to an atom of a different element. These can be made by igniting the element in the atmosphere.
S+O2→SO2
Trioxide is an oxide that comprises three oxygen atoms, each linked directly to an atom of a different element. The most important trioxide is SO3
2SO2+O2→2SO3
It is sulfuric acid's anhydride.
SO3+H2O→H2SO4
Trioxides are also produced by the element’s selenium and tellurium.
Also read -
Oxide's Chemical Formula
A negatively charged oxygen atom is called an oxide ion. It has gained two electrons from another atom in this way. O2– is the oxide symbol.
12O2(g)+2e–→O2–
Classification of Oxides
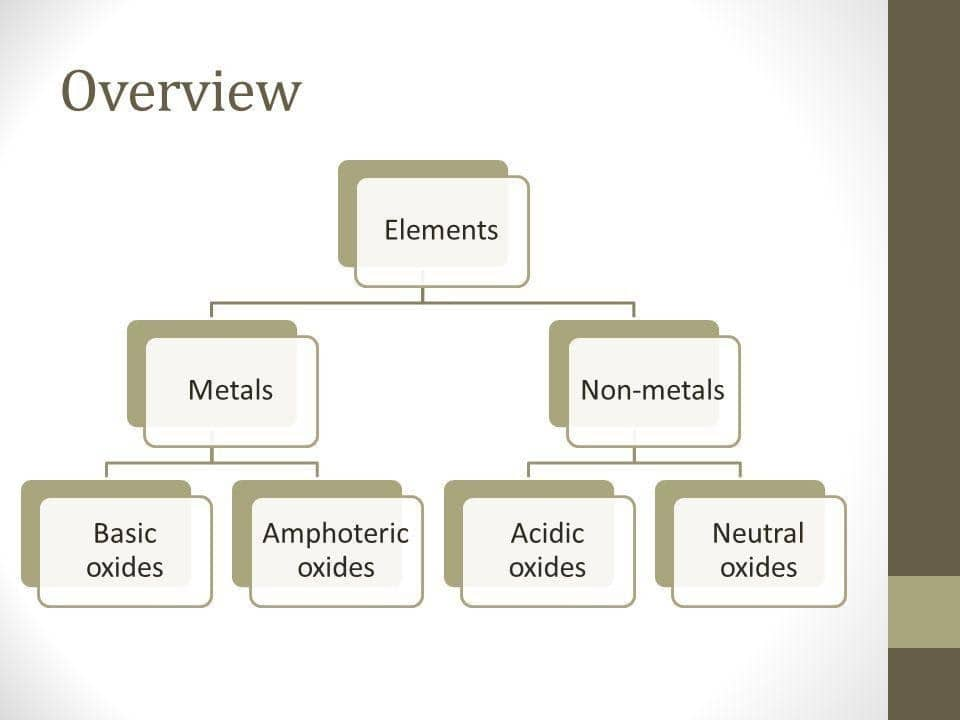
Oxides are divided into two categories:
Simple oxides are oxides that contain only the number of oxygen atoms permitted by the metal's usual valency. Acidic, basic, neutral, and amphoteric oxides are the four types of simple oxides. Simple oxides include magnesium oxide and aluminium oxide, to name a few.
Oxides of Mixed Composition
Mixed oxides are oxides that are made up of two comparable oxides. The elements in the two simple oxides could be the same or different. Let's go through mixed oxides in greater detail. When lead dioxide (PbO2) and lead monoxide (PbO) are combined, Red Lead is formed (Pb3O4).Ferric oxide (Fe2O3) and ferrous oxide (FeO) combine to generate Ferro-Ferric Oxide, a mixed oxide (Fe3O4). Magnetic Oxide is another name for this substance. Ilmenite is a ferrous oxide (FeO) and titanium oxide (TiO) mixed oxide (TiO2). A mixture of SrO and TiO2 is called a Strontium Titanate (SrTiO3).
Oxides' Characteristics
Different qualities can aid in the differentiation of the three types of oxides.
Oxide of Acid
Acidic oxide is an oxide that reacts with water to form an acid. Bases, such as sodium hydroxide, are neutralised by them. Acidic anhydrides are compounds that dissolve in water to produce acids. Sulphur dioxide, Cl2O7, and other compounds are examples.
Sulphur trioxide (SO3), for example, dissolves in water to form sulphuric acid (H2SO4).
SO3+H2O→H2SO4
Carbon dioxide (CO2) dissolves in water to form carbonic acid in the same way (H2CO3).
CO2+H2O→H2CO3
Acidity is a property of non-metallic oxides. Acids are formed when acidic oxides of non-metals dissolve in water. Non-metal acidic oxides change the colour of the water.
Oxide of Basic
Basic oxides are oxides that react with water to form a base. Examples are Na2O, CaO, and BaO. Acids such as hydrochloric acid are neutralised by them. They form bases when they dissolve in water. Basic anhydrides are what they're termed.
Na2O+H2O→ 2NaOH,
CaO+H2O→Ca(OH) 2
Metallic oxides are, in general, basic. The resulting solution turns red litmus blue when they react with water. Basic oxides are oxides that fall into this category.
Oxide of Amphoteric
Metallic oxides with both basic and acidic characteristics are known as amphoteric oxides. They produce salt and water when they react with an acid, demonstrating basic characteristics. However, when they react with alkalis, they produce salt and water, both of which have acidic qualities.
ZnO+2H2O + 2NaO→ Na2Zn(OH)4 + H2
ZnO+2HCl→ZnCl2+H2O
Another example is aluminium oxide, which reacts with both alkalis and acids.
When aluminium oxide reacts with hydrochloric acid, it becomes a basic oxide.
Al2O3(S)+6HcL(aq) →2AlCl3(aq)+ 3H2O
Aluminium oxide + Hydrochloric acid→ Aluminium chloride + Water
Water
When aluminium oxide reacts with sodium hydroxide, it forms an acidic oxide.
Al2O3(S) +2NaOH(aq) → 2NaAlO2(aq) + H2O(l)
Aluminium oxide + Sodium hydroxide → Sodium Aluminate + Water
Oxides with a neutral pH
Some oxides are neutral in nature, meaning they are neither acidic nor basic. Neutral oxides are the name given to such oxides. Nitrous oxide and carbon monoxide are examples of neutral oxides. Oxides in a Period and Group have acidic and basic natures. With an increase in the electropositivity of metal producing oxide, the basic character of the oxides normally increases. All group 1 oxides are basic, while group 17 oxides are acidic. The basic nature of oxides rises as they progress in the group, whereas the acidic nature decreases.
The basic nature of oxides steadily declines with time, whereas the acidic nature gradually increases. When a single element generates a large number of oxides, the acidic nature of the compound increases as the number of oxygen atoms grows. Caesium oxide is the most basic oxide. The strongest base is calcium hydroxide. Chlorine heptoxide is the most acidic oxide. The most powerful acid is perchloric acid.
Also, students can refer,
- NCERT solutions for Class 10 Science Chapter 2 Acids, Bases and Salts
- NCERT Exemplar Class 10 Chemistry Solutions Chapter 2 Acids, Bases and Salts
NCERT notes Class 10 Chemistry Chapter 2 Acids, Bases and Salts
Oxides of metals
Metal oxides are crystalline solids with a metal cation and an oxide anion. Salts are formed when they react with acids, and bases are formed when they react with water.
Metal Oxide Preparation: Metal oxides can be prepared in two ways:
Using the direct way
Method of indirection
In the direct method, metals react with oxygen to form metal oxides.
Example: 2Ca+O2 → 2CaO
2Mg+O2 → 2MgO
Thermal breakdown of carbonate salts, hydroxides, and nitrates is used in the indirect technique.
Example:
CaCO3 → CaO + CO2
2Pb(NO3) 2 → 2PbO + 4NO2 + O2
Metal Oxides and Acids Reaction:
When metal oxides react with acids, salts are formed. Magnesium oxide, calcium oxide, Sodium oxide, and potassium oxide are examples.
MgO+2HCl→MgCl2+H2O
CaO+H2SO4→CaSO4+H2O
Metal Reactions with Metal Oxide:
This reaction appears to be a ‘competition' for oxygen atoms between two metal atoms. The oxygen will be removed from the less reactive metal oxide by the more reactive metal atoms. The oxygen is transferred from the less reactive metal oxide to the more reactive metal as a result of this process. The more reactive metal always pulls oxygen away from the less reactive metal oxide, according to the rule.
Mg+CuO→Cu+MgO
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: