Coordination Compounds - Notes, Topics, Formula, Books, FAQs
In this chapter, you will study about the concepts of coordination compounds. These compounds are basically the compounds of the d-block elements. Several daily things like ruby, emerald, etc. as shown in the figure, are some common examples of this category. These compounds have major importance in industries, metallurgy, pigment(chlorophyll), etc.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Important Topics
- Overview Of The Chapter- Coordination Compounds
- Some Important Terms In Coordination Compounds
- Isomerism In Coordination Compounds
- Valence Bond Theory
- Crystal Field Theory
- Colour In Coordination Compounds
- Importance And Applications Of Coordination Compounds
- How To Prepare For Coordination Compounds?
- Prescribed Books
This article will help you with all the information related to this chapter. You will also get help about how to prepare for this chapter and the best-prescribed books.
Important Topics
Coordination Compounds:
Coordination Compounds , also known as complex compounds or addition compounds. All such types of compounds involve a central metal atom or `ion that is bonded to a number of molecules or ions called ligands. Examples of complex ions such as [Fe(CN)6]4– of K4[Fe(CN)6] do not dissociate into Fe2+ and CN– ions.
Werner's Theory:
Werner's Theory about coordination compounds states the reaction that the metallic ions undergo with the ligands to form a complex structure. The theory basically aims at the dual representation of valency, which may be termed primary and secondary. The metal oxidation valency signifies the primary valency of the metal and is ionizable, which is satisfied by the negative ions.
Valence Bond Theory Of Coordination Compounds:
Valence Bond Theory is a theory that explains the structure and magnetic properties of coordination compounds. According to this theory, the metal atom or ion under the influence of ligands can use its (n-1)d or nd orbitals along with its ns and np for hybridization to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar and so on.
Crystal Field Theory(CFT):
The Crystal Field Theory (CFT) is an electrostatic model which considers the metal-ligand bond to be ionic arising purely from electrostatic interactions between the metal ion and the ligand. Ligands are treated as point charges in the case of anions or point dipoles in the case of neutral molecules.
Factors Affecting The Stability Of Complexes:
The Stability Of Complexes is affected by various factors such as the stability of complex compounds increases as the oxidation state of the central metal atom increases, it increases as the charge on the central metal atom increases, increases as the charge density on the central metal atom increases, also increases as the electronegativity of the central metal atom increases. Chelating ligands which form 5-6 membered ring complex compounds are far more stable than any other complex compound.
Isomerism In Coordination Complexes:
Isomerism In Coordination Complexes is a captivating aspect of coordination chemistry that reveals the intricate ways in which ligands can arrange themselves around a central metal ion. This phenomenon leads to compounds that, while sharing the same molecular formula, exhibit distinct structural and spatial configurations.
Organometallic Compounds:
Organometallic Compounds are a class of chemical species defined by the presence of at least one bond between a carbon atom and a metal atom. Species of this kind are of interest and show properties that clearly distinguish them from organic and inorganic pure compounds.
Pi - Complex:
Pi- Complex coordination compounds are a class of chemical species formed through the interaction of transition metal ions with pi-electron systems, such as alkenes, alkynes, and aromatic compounds. These are the compounds of metals with alkenes, alkynes, benzene, and other ring compounds. In these complexes, the metal and ligand form a bond that involves the π electrons of the ligand.
Overview Of The Chapter- Coordination Compounds
Werner's theory of coordination compounds: Werner studied a large number of coordination compounds, their properties, and their structures. Based on his studies, he proposed a theory known as Werner's theory. the main postulates of his theory were as follows:
- In complex compounds, central metal shows two types of valencies i.e. primary and secondary valency.
- The primary valency is ionizable and is satisfied by the negative ions.
- The secondary valency is satisfied by neutral molecules or negative ions.
- Because of this secondary valency, ions or ligands bind to the central metal atom in a specific arrangement and thus these molecules have a shape.
Some Important Terms In Coordination Compounds
- Coordination entity: When the central metal atom is surrounded by ions or ligands and makes a complex, then it is known as the coordination entity. For example, [PtCl2(NH3)2].
- Central atom: It is a metal atom to which all the ions or groups are bonded in the complex compound. For example, in [PtCl2(NH3)2], Pt is the central atom.
- Ligands: The ions or groups that are bonded to the central metal are known as ligands. These ligands may be ions or neutral molecules. For example in [PtCl2(NH3)2], Cl and NH3 are ligands.
- Coordination number: The total number of ligands bonded to the central metal atom is known as the coordination number. For example, in [NiCl2(H2O)4], the coordination number for this complex is 6.
Isomerism In Coordination Compounds
Isomerism is the concept in which two or more compounds have the same chemical formula but they differ in their physical and chemical properties. In coordination compounds, this isomerism is of two types viz:
Stereoisomerism
- Structural isomerism
Stereoisomerism: Stereoisomerism is further classified into two categories:
(i) Geometrical isomerism: This isomerism arises when the ligands are bonded in different geometric arrangements. For example
(ii) Optical isomerism: This isomerism is the case when two isomers are mirror images of each other and these images are not superimposable to each other. These isomers are also known as enantiomers.
Structural isomerism: Structural isomerism is further classified into four categories:
(i) Linkage isomerism: This isomerism occurs in coordination compounds in which the ambidentate ligands are present. For example, in the case of thiocyanate ligand NCS-, this ligand can bind to the central metal atom either through the nitrogen side or through the sulfur side and give two linkage isomers.
(ii) Coordination isomerism: This kind of isomerism arises when the interchange of ligands between the cationic and anionic species takes place. For example [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6].
(iii) Ionization isomerism: This type of isomerism occurs when the counter ion itself is a potential ligand and can replace a ligand from the entity. For example [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4.
(iv) Solvate isomerism: This type of isomerism is similar to ionization isomerism. The solvate isomers differ in the way of the water molecule is present as a ligand or simply as a free molecule. For example [Cr(H2O)6]Cl3 and [Cr(H2O)5Cl]Cl2.H2O.
Valence Bond Theory
According to this theory, the central metal under the influence of the ligands uses its orbitals to form the complex molecule. Because of this bonding orbital, the coordination entity arranges itself in a definite shape and thus they have a geometry like tetrahedral or octahedral, etc. When the metal is using its inner orbitals, then the complex molecule is known as inner orbital or low spin complex and if the metal is using its outer orbitals for hybridization, then the complex is known as outer orbital or high spin complex.
Limitations of VBT: Although VBT could explain the formation, structure, and magnetic behavior of the complex compound it had various limitations as follows:
- It has a number of assumptions.
- It does not explain the color of coordination compounds.
- It does not give any information about the thermodynamic and kinetic stabilities of complex compounds.
- It does not differentiate between strong and weak ligands.
- It does not give a quantitative interpretation of magnetic data.
Crystal Field Theory
Crystal field theory assumes that both the central metal and the ligands are point charges and the interaction between them is completely electrostatic. The five d-orbitals in the metal are of the same energy, but when these orbitals are surrounded by the negatively charged field of ligands then this degeneracy is broken. This breaking of the degeneracy of orbitals occurs in two ways as follows:
- Crystal field splitting into octahedral coordination entities
When a central metal atom is surrounded by ligands then the degeneracy of the d-orbitals is removed. Basically, the orbitals dx2-y2 and dz2 point towards the axes along the direction of the ligand and thus experience more repulsion whereas the other orbitals dxy, dyz and dxz are between the axes and thus experience less repulsion. Thus, in this way, these orbitals lose their degeneracy and distribute themselves into two groups of orbitals, i.e, t2g set of lower energy and eg set of higher energy. The energy separation between these two sets of orbitals is denoted by $\Delta _{o}$.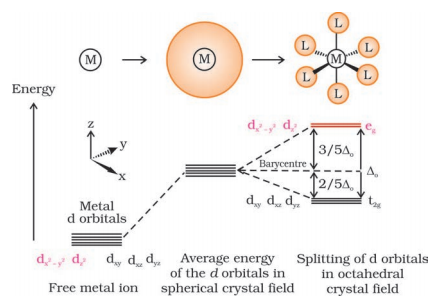
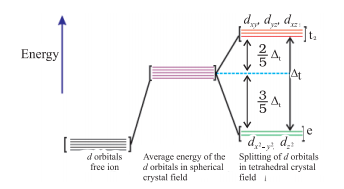
- Crystal field splitting into tetrahedral coordination entities
In tetrahedral splitting, this splitting is inverted. In this case, the energy gap is also smaller than octahedral splitting. Thus,
$\Delta _{t}\, =\,(4/9) \Delta _{o}$
The picture given below will show every detail related to tetrahedral splitting.
Colour In Coordination Compounds
The coordination compounds always have the property to exhibit colors. This is possible only because the compounds have the tendency to absorb some wavelength of light and emerge the rest of the light. In this way, the color of the coordination compounds is complementary to the wavelength that it has absorbed. Below is the table which gives the relationship between the absorbed light and colour of the coordination compounds.
Importance And Applications Of Coordination Compounds
Coordination compounds are present in many things like plants, minerals, etc. They are widely used in analytical chemistry, metallurgy, industry, etc. Some of the important applications of coordination compounds are as follows:
- Coordination compounds like Na2EDTA are used for the detection of the hardness of the water.
- The extraction processes of gold and silver are done by making use of the coordination compounds.
- Coordination compounds also have major importance in biological systems. For example, chlorophyll, a pigment for photosynthesis is a coordination compound of magnesium.
- Coordination compounds are used as catalysts for various industrial processes.
How To Prepare For Coordination Compounds?
This chapter is part of Inorganic chemistry. It is completely theory-based. You are not supposed to memorize any formulas and numerical practice to get a good hold on this chapter.
First, you must have complete knowledge of the Atomic Structure chapter. For this, you must go through chapter 3 of the NCERT book 11th class part 1 thoroughly.
You must deeply observe how and why the properties of elements like atomic radius, ionization enthalpy, electron gain enthalpy, etc. follow some general trends.
In these properties, there are also some exceptional cases that exist that you must understand, for example, why the oxygen atom has a bigger size than the nitrogen atom or why the electron gain enthalpy of chlorine is more than fluorine.
Prescribed Books
For this chapter, first, you need to finish the theory thoroughly from the NCERT book and then simultaneously solve the examples and questions given in the book. Apart from this, if you want to prepare for the advanced level for competitive exams like JEE and NEET, you must read the book - O.P. Tandon. Meanwhile, in the preparation, you must continuously give the mock tests for better understanding. Our platform "entrance360" will help you with a variety of questions for deeper knowledge and it will also provide you with concept videos, articles, and mock tests for better understanding.
Frequently Asked Questions (FAQs)
A ligand is a molecule or ion that can donate at least one pair of electrons to a central metal atom or ion to form a coordination complex. Ligands can be classified based on their denticity (the number of donor atoms), such as monodentate (one donor atom), bidentate (two donor atoms), and polydentate (multiple donor atoms).
Naming coordination compounds follows specific rules established by IUPAC. The name typically starts with the ligands listed in alphabetical order, followed by the name of the central metal ion. Prefixes (mono-, di-, tri-, etc.) indicate the number of each type of ligand, and oxidation states of the metal are indicated in Roman numerals in parentheses.
Coordination compounds have various applications, including in catalysis, as dyes and pigments, in medicine (e.g., chelating agents), in electrochemistry, and as materials in nanotechnology. They also play critical roles in biological systems, such as hemoglobin and chlorophyll.
Chelation refers to the process where a multidentate ligand forms multiple bonds with a central metal ion, creating a stable ring-like structure. Chelating agents are important for removing toxic metals from the body, enhancing the bioavailability of essential nutrients, and stabilizing metal ions in various applications.
The geometry of a coordination compound can often be predicted based on the coordination number and the type of ligands involved. For instance, a coordination number of 4 usually leads to a tetrahedral or square planar geometry, while a coordination number of 6 typically results in an octahedral geometry, as based on VSEPR (Valence Shell Electron Pair Repulsion) theory.
Also Read
20 Dec'24 05:48 PM
18 Nov'24 03:03 PM
04 Nov'24 11:17 AM
21 Oct'24 06:24 PM
21 Oct'24 06:14 PM
21 Oct'24 04:19 PM
17 Oct'24 06:11 PM
17 Oct'24 06:00 PM