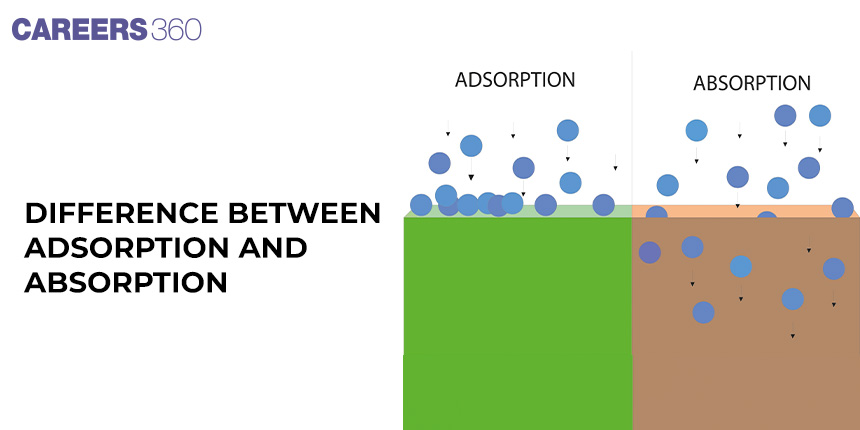
Difference between Adsorption and Absorption.
Introduction
Generally, adsorption and absorption are the two major processes that deal with the surface sticking of a molecule from a substance. As a matter of fact, it is very imperative to know what separates these in different scientific and industrial applications. Imagine you are in a crowded market and you can totally feel that all sorts of food smells from stalls are around. Some smells can stick on the fabric while some soak through and stick on the skin. Basically, these are processes whereby molecules stick to a surface with scent in varied ways.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Introduction
- Adsorption Mechanism
- Thermodynamics of Adsorption
- Adsorption: An Introduction
- Adsorption Mechanism
- Applications of Adsorption
- Summary
Adsorption Mechanism
Adsorption is the procedure through which fluid molecules, either in a liquid or vapor state, stick to the surface of a solid or liquid. The force tending to hold a liquid in contact with a solid surface is really a stress and is then referred to as an interaction. The interaction at the surface with solids and liquids is usually that which is caused by intermolecular forces of attraction which are referred to as Van der Waals forces, electrostatic interactions, and hydrogen bonding. Its extent depends on factors such as surface area, temperature, and pressure.
Factors Affecting Adsorption
There are a number of factors influencing the extent of adsorption, among them:
- Surface Area: The larger the surface area, the more adsorption sites become available.
- Temperature: In general, the increase in temperature of the system decreases adsorption to some extent.
- Nature of Adsorbate and Adsorbent: Affinity between different substances.
Thermodynamics of Adsorption
Almost invariably, adsorption is associated with changes in entropy and enthalpy. The value of ΔG tells whether adsorption is favorable or not, that is ΔG < 0 for favorable adsorption; otherwise, it is not favorable. Thus the greater the value of ΔG, the greater is the extent of adsorption.
Adsorption: An Introduction
The process of adsorption is the transfer of molecules of the fluid to the surface of a solid or liquid body, and the spreading over that body in a monolayer or a multilayer. This phenomenon forms the ground for lots of very important industrial processes, including catalysis, chromatography, and treatment of wastewater. The mechanism of adsorption itself, together with the factors influencing it, is instrumental in the optimization of all these procedures in view of the further development of materials with altered adsorption-property materials.
Adsorption Mechanism
Adsorption is brought about by the intermolecular forces existing between the adsorbate, the molecules belonging to the fluid phase, and the adsorbent being basically solid or liquid in character. The forces involved include Van der Waals forces, electrostatic interactions, and hydrogen bonding; therefore, when the adsorbate molecules come in contact with the adsorbent surface, they will bind to it because the free energy for the system will decrease.
Adsorption Influencing Factors
There are various factors that serve to affect the total uptake and efficiency of adsorption. These are:
- Surface Area: The more the surface area, the more the number of sites available for adsorption. This will, therefore, aid in the amount of adsorbate that can be adsorbed.
- Temperature: Adsorption is commonly a diminishing function of temperature since a change in the rate of equilibrium between the adsorption and the corresponding desorption usually occurs.
- Chemical and Physical Nature of the Adsorbate and Adsorbent: The chemical and physical nature of the adsorbate and adsorbent dictates interaction, thus based on the chemical and physical properties. A good example of an application in which the interaction is stronger with the adsorbate in a polar adsorbent is found in this referencing application.
Applications of Adsorption
There are numerous applications of adsorption. Adsorption is thus used in the outlined fields:
- Environmental Remediation: Adsorption is applied to aid in the removal of pollutants in both wastewater and air streams.
- Separation Processes: In this referencing technique, it is applied in pharmaceutical and chemical separation in adsorption chromatography.
- Gas Purification: Process by which gas impurities are removed making them free from impurities through adsorption. Impurities are transferred into materials, thereafter ultra-high purity gases are produced from such material.
Recommended topic video on (Difference between Adsorption and Absorption)
Some Solved Examples
Example 1
Question: Adsorption is a:
1. Colligative property
2. Bulk phenomenon
3. Oxidation process
4. Surface phenomenon (correct)
Solution:
Adsorption occurs at the surface of a solid where the solid has the tendency to attract and retain the molecules of the phase it contacts. These molecules remain at the surface and do not penetrate the bulk of the solid. Therefore, adsorption is a surface phenomenon. The correct answer is option 4.
Example 2
Question: The substance that gets adsorbed on the surface of the solid is called:
1. Adsorbate (correct)
2. Adsorbent
3. Micelle
4. Inner phase
Solution: The molecular species or substance that accumulates at the surface of a solid is termed an adsorbate. The material on the surface of which adsorption takes place is called the adsorbent. Hence, the correct answer is option 1.
Example 3
Question: The correct match between Item I and Item II is:
Item I:
A) Benzaldehyde
B) Alumina
C) Acetonitrile
Item II:
P) Mobile phase
Q) Adsorbent
R) Adsorbate
1. (A) → (Q); (B) → (P); (C) → (R)
2. (A) → (Q); (B) → (R); (C) → (P)
3. (A) → (P); (B) → (R); (C) → (Q)
4. (A) → (R); (B) → (Q); (C) → (P) (correct)
Solution: In the context of adsorption:
- Benzaldehyde is an adsorbate.
- Alumina acts as an adsorbent.
- Acetonitrile functions as a mobile phase.
Therefore, the correct matching is:
- (A) Benzaldehyde → (R) Adsorbate
- (B) Alumina → (Q) Adsorbent
- (C) Acetonitrile → (P) Mobile phase
The correct answer is option 4.
Summary
Adsorption is a universal process in which molecules attach to a solid or liquid interface due to the action of intermolecular forces. In addition, the process mechanism and the parameters influencing the process benefit from operating data in further developing the optimization of industrial processes or the design of new materials with increased adsorption performance.
Also Read
19 Feb'25 10:44 AM
17 Dec'24 10:24 AM
17 Dec'24 10:21 AM
29 Nov'24 12:13 PM
30 Sep'24 02:31 PM
30 Sep'24 11:46 AM
26 Sep'24 06:10 PM
26 Sep'24 05:53 PM