Difference Between Element and Compound - Definition, Example, FAQs
In this article we will understand the difference between element and compound and learn how to differentiate between elements and compounds. We need to first understand the definition of element and compound, before we start to differentiate between element and compound.
This Story also Contains
- Element
- Compound
- Difference between element and compound with example
Element
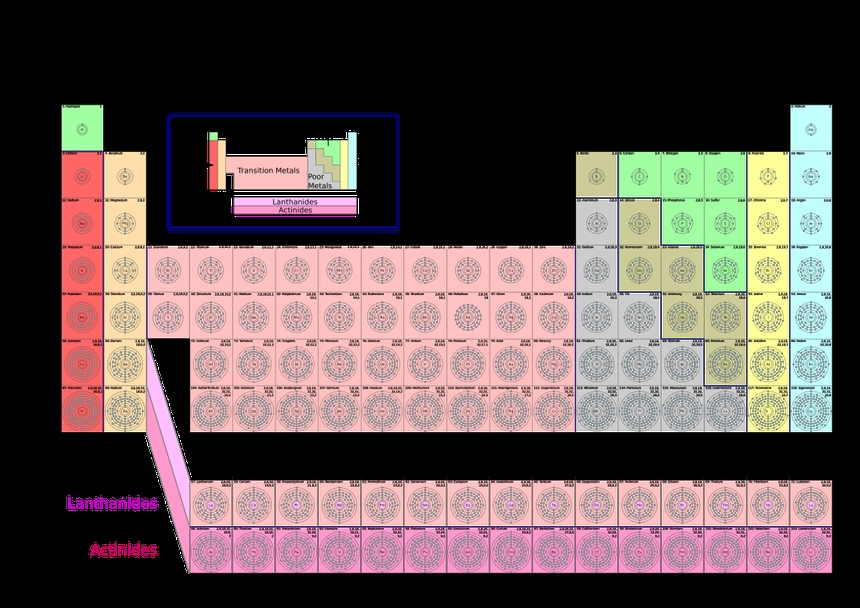
An element can be said to be a substance that consists of only one type of atom. An example is that of the carbon atom. Another example can be hydrogen atoms which have a single proton as well as a single electron in their outermost orbitals. Whenever there is a change in the number of protons of an atom, the type of element changes like in nuclear reactions. An element is a substance in which the atoms have the same atomic number or same number of protons. Consequently, this means that the atoms of an element have the same number of electrons. We cannot break down elements which in turn makes elements the simplest substance.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
It is obvious that all the atoms of an element must have the same number of protons, although their number of neutrons can differ from element to element or even atom to atom. Which means masses of atoms of a certain element can differ as mass of an atom is the sum of the number of protons and number of neutrons. Such elements with the same number of protons (atomic number) but different numbers of neutrons (mass number) are called isotopes.
For example isotopes of hydrogen are protium ( with atomic number one and mass number one), deuterium (with atomic number one and mass number two),Tritium (with atomic number one and mass number 3), isotopes of carbon, isotopes of phosphorus, isotopes of nitrogen etc. A component refers to a small portion of a larger entity that may or may not contribute to the larger entity’s properties. An element is made up of certain atoms with certain atomic numbers. This is the key difference between elements and components.
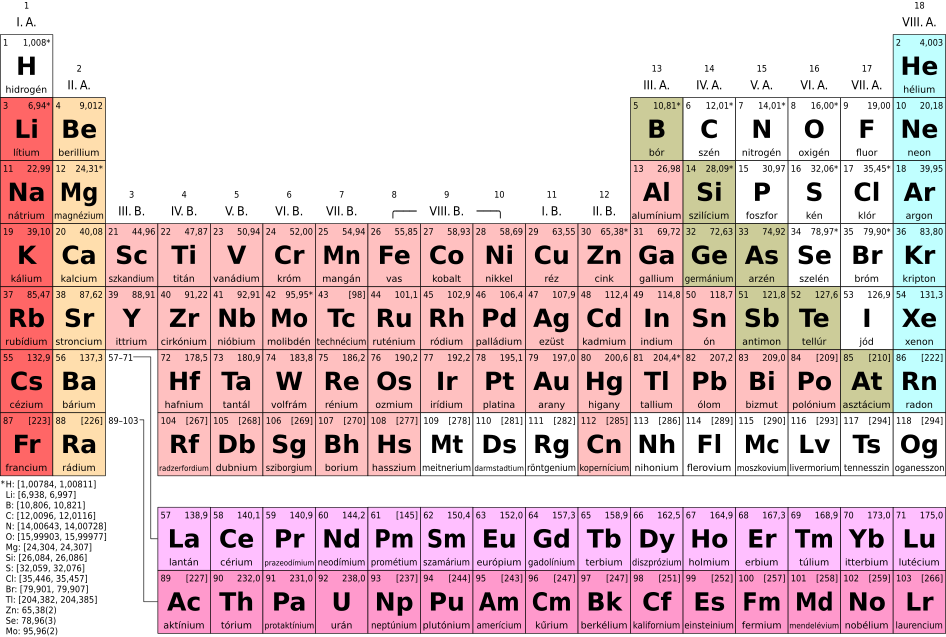
There are 118 elements in total. Some examples of elements are:
The W element is tungsten.
The C element is carbon.
B element is boron.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 1 Some basic concepts of Chemistry
- NCERT notes Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
Compound
A compound can be defined as a substance that forms due when two or more elements combine. These elements are different from one another. These elements come closer to interact through different forces and combine by formation of different bonds such as covalent bonds and electrostatic bonds. Electrostatic bonds are formed between atoms when electrons are shared between atoms and electrostatic force develops between ions. A mixture is formed due to physical mixing of different kinds of substances to form a certain type of substance.
Mixtures can broadly be classified as homogeneous and heterogeneous mixtures. Homogeneous mixtures have uniform concentration of solute throughout the mixture. Heterogeneous mixtures on other hand have non-uniform concentration of solute throughout the mixture. A compound formation occurs due to combination of different types of substances through a chemical reaction. An example of compound is carbon dioxide and water which are formed in the process of combustion. This is a key difference between elements and compounds and mixture.
A compound may or may not behave like any of the elements from which it is formed. For example, elements carbon (C) and oxygen (O) tend to be gases at room temperature, when the atmospheric pressure is normal and their product carbon dioxide is gas too at normal pressure. But if we look at the case of hydrogen element (H) and oxygen (O) element, their product water is liquid at normal pressure and room temperature. This indicates that a compound may or may not possess the same property as its components.
There are certain ‘special’ atoms of a few elements that do not bond with any other elements easily at normal conditions. This means they do not form compounds at all and thus are called noble. These unreactive elements are called inert or noble gases like neon, argon, helium, xenon, krypton, and radon which reside in the 18th group of the periodic table. After discussing elements and compounds we can discuss element and compound differences in detail.
Difference between element and compound with example
To distinguish between element and compound, it is best to decide to organize some differentiating criteria on which we could differentiate an element or compound.
S.No. | Criteria | Element | Compound |
1. | Definition | An element can be said to be a substance that consists of only one type of atom. | A compound can be defined as a substance that forms due when two or more elements combine. |
2. | Overall total | There are a total of 118 elements. | The number of compounds existing is immeasurable. |
3. | Typing criteria | Elements are generally classified as nonmetals, metalloids and metals on the basis of various physical and chemical properties. | Compounds can be classified as ionic, metallic, covalent etc. based on the type of interaction between atoms. |
4. | Representation | These are represented by symbols and atomic numbers. A symbol may or may not correspond to the universal name of an element. For example, the symbol for sodium is Na. | Compounds exist due to the fact that atoms come together. Thus, compounds are represented by writing symbols of constituents’ atoms of different elements together in a combined chemical formula. Example: Na2CO3, NaOH, CO2 etc. |
5. | Distinguishing factor | Elements are distinguished by their atomic number. Example: atomic number of hydrogen is 1 while atomic number of boron is 5. | Compounds can be distinguished by their respective ratio of atoms of a certain element. Example CO2 (ratio of C:O = 1:2) is different from CO (ratio of C:O = 1:1). |
6. | Property and composition | These consist of only one type of atom which has its characteristic atomic number. | These consist of different types of atoms of different elements which combine in different ratios. |
7. | Breakdown capability | Elements cannot be broken down in simpler substances | Compounds can be broken down into molecules of one or many elements. |
8. | Examples | Examples include w element, b element, c element, o element etc. | Examples include nitrogen dioxide, water, carbon dioxide, calcium hydroxide etc. |
The above table can be of immense importance for understanding element vs compound battle.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
