Difference Between Molar Mass and Molecular Mass with FAQs
Molar mass
Molar mass is also known by the name relative molar mass which can be defined as the smallest mass unit of any compound with respect to the one twelfth of the mass of a carbon atom.
What is the molar mass unit?
Unit of molar mass can be defined by two units one is standard unit and other one is S.I. unit. Standard unit of molar mass is gmol-1 and S.I. unit is given by Kgmol-1, S.I. unit of molar mass is rarely seen.
Molar mass can also be defined on the basis of mole concept where mole of any substance is defined as a substance or particle can be defined as containing exactly NH3=14+3×1=17u particles which may be atoms, molecules or ions where 6.02214076×1023 is known as Avogadro's number. Mole can be represented by its symbol called mol. It is generally described as the unit of measurement for amount of substance in SI where SI stands for International System of units. Mole can be easily defined on the basis of Avogadro’s number.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Molar mass
- Difference between molar mass and atomic mass
- Molecular mass
- Difference between molar mass and molecular mass:
The number of atoms in 12g (0.012 kg) of isotope of carbon i.e. 12C equals the number of particles in 1 mole of the material. One of the most crucial facts to remember is that any substance mole always contains the same amount of entities, regardless of the substance.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Molar mass formula
Calculating the atomic, molecular, and formula masses of atoms, molecules, and other compounds is fine, but since we can't weigh a single particle, these masses are only of limited utility. We must express chemical quantities at the macroscopic level to make mass measurements useful. Molar mass, or the mass in grams of one mole of a substance, serves as a link between the particle and macroscopic levels. The units of molar mass are grams per mole, as defined by its definition. The defining equation of molar mass in mathematics is
Molar mass = mass/mole = g/mol
Carbon-12 is directly or indirectly related to the definitions of atomic mass, mole, and molar mass. This leads to two key points which are described as:
- The atomic mass of one carbon-12 atom is exactly 12 atomic mass units.
- One mole of carbon-12 atoms has a mass of exactly 12 grams, and its molar mass is also exactly 12 grams per mole.
Difference between molar mass and atomic mass
Carbon-12's atomic mass and molar mass are quantitatively equivalent. Only the units are different: atomic mass is measured in atomic mass units, whereas molar mass is measured in grams per mole. The atomic and molar masses of elements, molecular masses and molar masses of molecular substances, and formula masses and molar masses of ionic compounds all have the same relationship.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 1 Some basic concepts of Chemistry
- NCERT notes Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
Molecular mass
The molecular mass of a substance is defined as the sum of the mass of every single molecule present in it. We can easily calculate it by just adding the atomic mass units of the individual atoms in order to calculate the molecular mass of a molecule. Dalton is the most common unit of molecular mass, and it is symbolized by Da and one Dalton is equal to one unit i.e. 1Da=1u.
Molecular mass formula
The molecular mass can be easily calculated by multiplying the number of atoms in each molecule to the given molar mass of atoms. The quantity of mass associated with a molecule is known as its molecular mass. The mass of each atom multiplied by the number of atoms of the element present in the molecule can be computed. By taking the example of water we can define a molecular mass formula: water generally contains two atoms of hydrogen and one atom of oxygen. The average atomic mass of hydrogen multiplied by two plus the atomic mass of oxygen equals the mass of a water molecule. The molecular mass of elements is determined by the atoms that make up the molecule.
Related Topics Link, |
Difference between molar mass and molecular mass:
Molar mass and molecular mass can be differentiated on the basis of following factors:
1. Molar mass is generally referred to with the term mole whereas molecular mass is associated with mass of molecules present in a compound.
2. Molar mass is also known by the name molecular weight whereas molecular mass is known to us for determining the mass of a single molecule.
3. Molar mass is expressed in units like in g/mol for higher calculations while molecular mass is expressed in amu i.e. atomic mass unit.
4. Molar mass is generally defined as the mass of Avogadro number of atoms, molecules or compounds while molecular mass is defined as the sum of atomic masses of all the atoms which are present in the given molecule or compound.
5. In case of molar mass the measurement is given to compounds, atoms or molecules while in case of molecular mass we only determine molecules.
6. Molar mass is generally less accurate as compared to molecular mass while molecular mass is accurate and helps in higher calculations.
Is molar mass and molecular mass are same?/ Is molar mass and molecular mass same and the main examples of molar mass and molecular mass can be shown as follows:
Molar mass: The example of molar mass can be considered by taking the example of mass of 1 mole of oxygen which is 15.9994 grams which is exactly equal to molar mass which is given by 15.9994 g/mol.
Molecular mass: Molecular mass of water is just add up the hydrogen and oxygen atoms by summing them up i.e. in case of H2O hydrogen atoms are available and one oxygen atom is present and we know that the mass of a hydrogen atom is 1 u and mass of one oxygen atom is 16 u. Now molecular mass of water is calculated by:
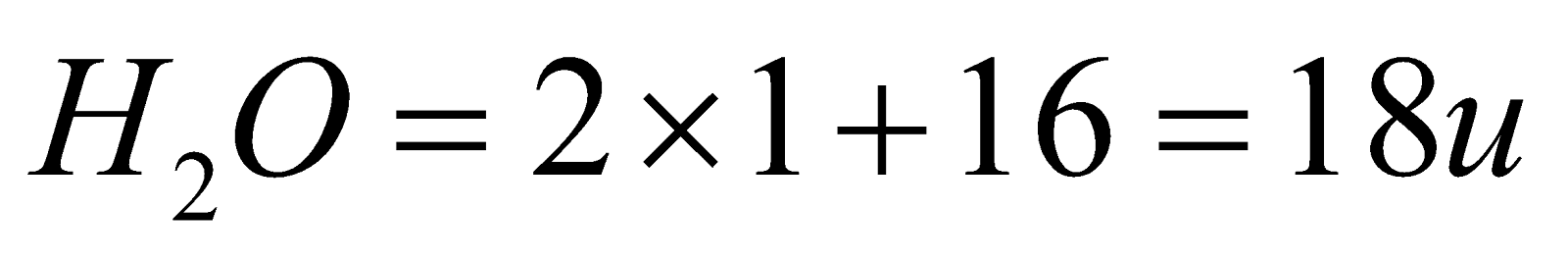
Hence on the basis of these points we can easily differentiate between molar mass and molecular mass.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Avogadro's number or Avogadro constant is generally gives us the relation between the number of constituent molecules, atoms or ions in a given sample with their corresponding volume given in that sample.
In chemistry atomic mass is very important as it provides us the connection between moles and masses i.e. number of atoms in any substance that’s why it get greater importance as we come to know about how many atoms are present in any compound through its atomic mass.
Mole can be represented by its symbol called mol. It is generally described as the unit of measurement for the amount of substance in SI where SI stands for International System of units. Mole can be easily defined on the basis of Avogadro’s number.
Equivalent mass is defined as the molar mass of substance which is divided by n number of equivalents present in the substance.
Also Read
30 Dec'24 11:52 PM
17 Dec'24 10:32 AM
09 Dec'24 10:47 AM
09 Dec'24 10:45 AM
18 Oct'24 11:04 AM
18 Oct'24 10:57 AM
18 Oct'24 10:52 AM