Difference Between Orbit and Orbitals - Overview, Definition, FAQs
The difference between an orbit and an orbital. Students and professionals who are interested in chemistry should understand the basics of the subject. As electrons revolve around an atom's nucleus, their orbit is a fixed path. The orbit of an atom is therefore particularly large. Understanding the molecular orbital theory here is crucial. Despite many people thinking orbits and orbitals are very similar, it is important to know the difference between them.
This Story also Contains
- What Is The Meaning Of Orbit?
- Orbital Definition
- Difference Between Orbit And Orbital
- Orbit
- Orbitals
- Some Solved Examples
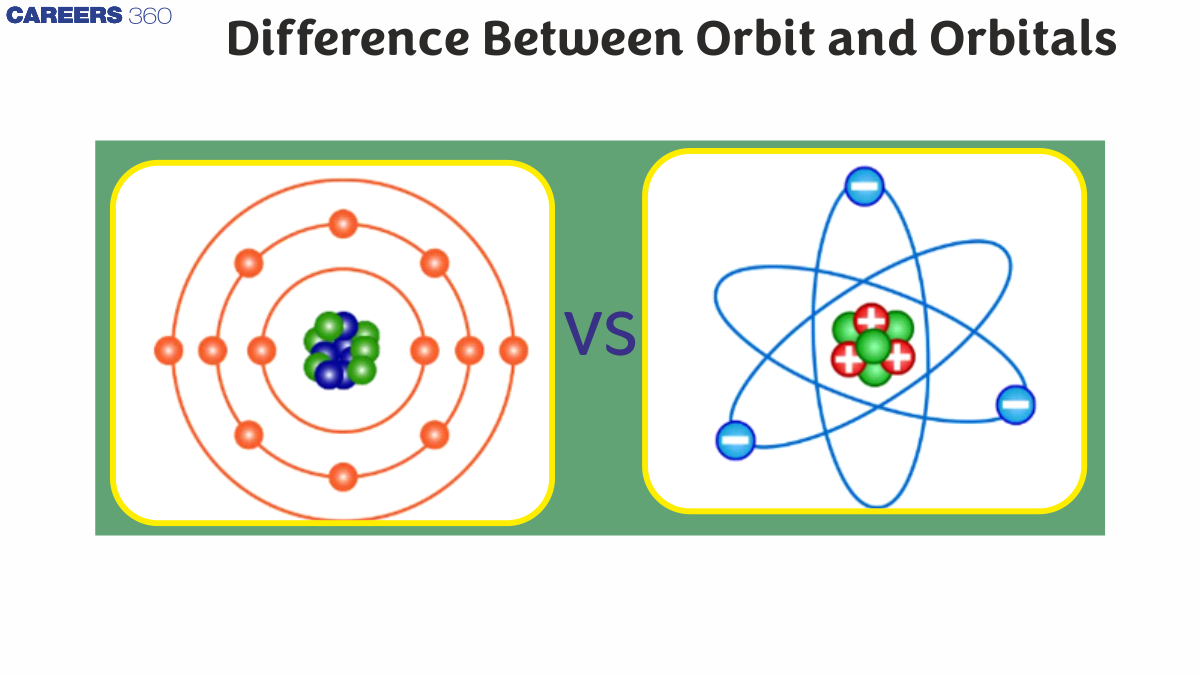
What Is The Meaning Of Orbit?
Orbit Definition: The orbit, meaning of a molecule in chemistry, is a definite path formed by regular rotations of electrons. Furthermore, electrons are pulled toward the nucleus by the pull of electrons. Furthermore, the first shell of an atom is only comprised of two electrons according to Bohr's model.
Orbital Definition
There is a large probability that an electron could be located in an uncertain area called an orbital. In addition, the space around the nucleus is three-dimensional, reflecting the orbital. Furthermore, it is also possible for the orbital to have different kinds of shapes. An orbital, on the other hand, is simply the probable region where one can expect to find the maximum density of electron presence within an atom. A body's orbit, meaning instead, contains only a certain amount of mass, while an electron's orbit contains both an atom and an electron. This is why Orbital and Orbit are two different things. Here is a comparison of Orbits and Orbitals so you can see how the two differ.
|
Related Topics link |
Difference Between Orbit And Orbital
|
Orbit |
Orbitals |
|
Electrons are represented by simple planar orbits. |
In a three-dimensional motion, an orbital describes the motion of an electron around the nucleus. |
|
As defined by electrons rotating around the nucleus, it represents the path that gets established. |
Orbitals are simply defined as the areas where electrons are likely to be found most frequently. |
|
The form of an orbital is circular |
The shape of an orbit can be spherical, bell-shaped, etc. |
|
The Heisenberg Uncertainty Principle does not apply to orbits since they claim the exact position of an electron. |
As electrons' exact locations are not represented by orbitals, Heisenberg's Uncertainty Principle certainly applies. |
|
It is possible to define orbits by letters such as L, M, N, etc. |
Orbitals can be defined by letters like f, s, p, and d. |
Also read :
Orbit
The magnetic field of an atom created by an electron is known as its orbit in chemistry. Also, an electron's orbit is simply a representation of an electron's location on a plane. In addition, it is the path whose establishment takes place because electrons revolve around the nucleus in a circular motion. It is impossible for an orbit to explain the shape of molecules. Because molecules have no directional properties, this is the case. Heisenberg's Uncertainty Principle is certainly violated by electrons.
Orbitals
An orbital in chemistry can be one of four different types. Chemical orbitals are distinguished by four types: sharp (s), principal (p), diffuse (d), and fundamental (f). Within each shell of the atom, there are certainly some combinations of orbitals. Furthermore, only the s orbitals are found in the n=1 shell. Additionally, there are s and p orbitals in the n=2 shell. Additionally, the n=3 shell contains orbitals of type, s, p, and d, while the n=4 up shell will contain these orbitals as well. An important point to note is that these orbitals belong to an empirical theory whose goal is to explain the observations of scientists regarding molecular structure and bonding. An orbital in chemistry is an illustration of two electrons located near the nucleus, represented as a wave function. Furthermore, the depiction of an orbital takes place in three dimensions in which electrons have a 95 percent probability of locating.
Some Solved Examples
Questions: Which of the following is true about an "orbit" in the Bohr model of the atom?
a) It is a three-dimensional region where electrons are likely to be found.
b) It is a fixed, circular path around the nucleus where electrons move. (correct)
c) It describes the probability distribution of an electron.
d) It represents a quantum mechanical model of electron behavior.
Solution:
In Bohr's model, electrons move in fixed, circular orbits around the nucleus at specific energy levels. These orbits are distinct paths, unlike the probability regions described in quantum mechanics.
Hence, the correct answer is option (b)
Questions: Which of the following best describes an "orbital"?
a) A specific path taken by an electron in an atom.
b) A fixed, circular trajectory followed by electrons.
c) A region in space around the nucleus where the probability of finding an electron is highest. (correct)
d) A definite location of an electron in an atom.
Solution:
An orbital in quantum mechanics refers to a region around the nucleus where there is a high probability of finding an electron. Orbitals do not represent a fixed path, but a probabilistic area.
Hence, the correct answer is option (c)
Questions: What distinguishes an orbital from an orbit?
a) An orbital is a fixed path; an orbit is a probabilistic region.
b) An orbital is described by quantum mechanics, whereas an orbit is based on classical physics.(correct)
c) An orbital is always circular, while an orbit is always elliptical.
d) There is no difference; both terms are used interchangeably.
Solution:
"Orbit" refers to the classical concept of a fixed, circular path for an electron, as per Bohr's model. "Orbital," however, is a quantum mechanical concept where electrons are found within regions of high probability, with complex shapes like s, p, d, and f.
Hence, the correct answer is option (b)
Practice More Questions With The Link Given Below
| Line spectrum of hydrogen practice questions and MCQs |
| Bohr's Model for the Hydrogen Atom practice questions and MCQs |
| Orbital frequency practice questions and MCQs |
Also read -
| NCERT Solutions for Class 12 Chemistry | NCERT notes Class 11 Chemistry |
| NCERT Solutions for Class 11 Chemistry | NCERT notes Class 12 Chemistry |
| NCERT Solutions for All Subjects | NCERT Notes For All Subjects |
Frequently Asked Questions (FAQs)
There is a maximum probability that an electron will be found in an orbital in an atom, so that defines an orbital. The surrounding three-dimensional space of the nucleus extends beyond it. Different kinds of orbits may have different shapes, such as sharp (s), principal(p), diffuse (d), and fundamental(f).
These subshells are called s, p, d, or f. The s-subshell can fit 2 electrons; p-subshell can fit a maximum of 6 electrons; d-subshell can fit a maximum of 10 electrons, and f-subshell can fit a maximum of 14 electrons.
Electrons revolve around the nucleus of an atom along a fixed path called an orbit. In the case of electrons, a nucleus-orbital (orbital of electrons) is the three-dimensional space around the nucleus in which the probability of finding electrons is highest (90-95%).
An orbit specifies the exact position of an electron within an atom, whereas an orbital does not specify exactly where an electron is located within an atom.
Some differences between orbits and orbitals are related to electron positions. Additionally, an orbit refers to exactly where an electron is located within an atom. An orbital, on the other hand, does not accurately portray the electron's location.
The orbit in chemistry refers to the path around the nucleus of an atom where electrons move in revolutionary motion. A simple planar representation of an electron is called an orbit. In addition, a circular motion establishes a path.