Difference Between Physical and Chemical Change - Definition, Characteristics, Examples, FAQs
Matter undergoes two different types of changes in nature: physical and chemical change. A physical change would affect a material's physical characteristics, while a chemical change impacts the chemical properties, as its names suggest. Many physical changes including heating and cooling are recoverable, whereas chemical changes are irreparable or only reversible with another chemical process.
This Story also Contains
- Definition of Physical Change :
- Definition of Chemical Change :
- Characteristics of physical change:
- Characteristics of chemical change:
Definition of Physical Change :
Physical transformation or change is a result in which a substance's physical qualities, such as shape, size, quantity, appearance, colour, state (i.e., solid, liquid, gas), and so on, change without affecting its molecular composition. These changes are inherently unstable, but they can be reversed with basic physical techniques. The same component or combination exists before or after the alteration, i.e. the object's fundamental physical appearance is preserved. Examples of physical changes comprises, wax melting, glucose dissolving in water, water bubbling, wood cutting, paper crumpling, and so on.

Also read :
Definition of Chemical Change :
Chemical change is explained as the method of rearranging or combining the molecules of one or even more substances to generate a new component. When a substance undergoes chemical transformation, its molecular character changes, and this is transformed into a new substance with a specific chemical structure. The development of energy, the formation of bubbles, and temperature variations are all examples of chemical change.
Alternatively, chemical change is called as a chemical reaction, in which the chemicals involved are referred to as reactants, as well as the reaction's outcome is referred to as a product. Because of the creation of the entire product, energy change is one of the characteristics of a chemical change. It is impossible to reverse the chemical change once it has occurred. For example, bleaching a stain, combining vinegar and baking soda, and so forth.
- Common Physical Changes :
Texture:
A physical alteration can modify the smoothness of a substance. A piece of wood that has been sanded, oiled, and polished, for example, will have a very different appearance than it had when it was rough.
Colour:
A substance's colour changing isn't always an indication of a chemical change. Changing the colour of a metal, for example, has no effect on its physical qualities. A colour change, on the other hand, is frequently an indication that a chemical reaction is taking place. The nature of the metallic component is not changed by painting the metal car whereas the physical appearance is altered.
Temperature:
Even though we cannot see change in temperature, it is a physiological response unless a change of condition occurs.
Shape:
An entity's shape can be modified while the object's molecular structure remains unchanged.
Change of state:
A physical change occurs when a substances changes its state. A multitude of physical qualities, such as viscosity and form, are changing in this scenario. When ice melts into water, it loses its solid form and becomes a viscous fluid.
Related Topics |
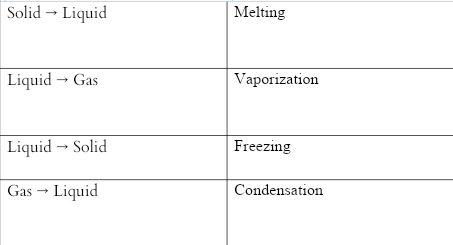
The common physical changes of state are:
Self-created using ms word tool
Common Chemical Changes :
Change in Temperature
Variation in temperature is major characteristic of a chemical change.
Change in colour:
Another sign that a chemical reaction is currently happening is a change in colour. For instance, observing the rusting of metal over time will reveal that the metal has changed colour and glowed yellow. This colour shift is the result of a chemical process.
Odor development:
A chemical reaction occurs when two or more chemicals or components are combined, and a fragrance or odor is produced. A chemical reaction has occurred when an egg begins to smell (a rotting egg, for example). A chemical decomposition has resulted in this.
Formation of precipitate:
One of the most common signals that a chemical reaction is taking place is the emergence of a precipitate. A precipitate is a substance that develops inside another solid or within a solution.
Characteristics of physical change:
Properties of physical change has three characteristics:
There is no formation of a new material.
The modifications are recoverable.
Both the product and the reactant have the same physical characteristics.
Characteristics of chemical change:
Properties of chemical change has four major characteristics:
A chemical reaction results in the formation of a new material.
The alterations are long-term.
The change is usually irreversible.
It also entails the absorption or release of energy, such as heat or light.
What is the difference between physical and chemical change?
Distinguish between physical and chemical change | |
PHYSICAL CHANGE | CHEMICAL CHANGE |
The molecules are reconfigured because of a physical change, but their true composition stays unchanged. | A chemical change causes a substance's atom configuration to totally change, resulting in the formation of a new compound. |
Physical change is only a short term transformation | A chemical change is a long-term transformation. |
Only physical properties, such as shape and size, are affected by a physical change. | Chemical changes the substance's physical and chemical qualities, as well as its makeup. |
A physical change includes little or no heat transfer. | Absorption and development of energy occur during a chemical process. |
Physical changes include the freezing of water, the melting of wax, the boiling of water, and so on. | Food digestion, coal combustion, corrosion, and other chemical changes are examples. |
Physical changes, in definition, do not necessitate the creation of energy. | The creation of energy is frequently involved in chemical transformations (which can be in the form of heat, light, sound, etc.) |
A physical change does not result in the formation of a new material. | One or even more new substances are always present when a chemical transition occurs. |
The physical change is reversible, which means the original material can be retained. | Chemical transformations are irreversible, which means that the original component cannot be restored. |
The molecules are reconfigured during a physical change, but their true composition remains unchanged. | A chemical change causes a substance's atom configuration to totally change, resulting in the formation of a new compound. |
Self-created using ms word tool
The above table shows the difference between physical change and chemical change.
Also read -
Frequently Asked Questions (FAQs)
Physical transformation or change is a result in which a substance's physical qualities, such as shape, size, quantity, appearance, colour, state (i.e., solid, liquid, gas), and so on, change without affecting its molecular composition. These changes are inherently unstable, but they can be reversed with basic physical techniques.
Chemical change is explained as the method of rearranging or combining the molecules of one or even more substances to generate a new component. When a substance undergoes chemical transformation, its molecular character changes, and this is transformed into a new substance with a specific chemical structure. The development of energy, the formation of bubbles, and temperature variations are all examples of chemical change.
Chemical change is usually accompanied by a change in colour, evolution of gas or formation of precipitate or the development of an odour. Rotting of eggs is an example for a chemical change having odour development.
Physical change is only a short-term transformation whereas a chemical change is a long-term transformation. Only physical properties, such as shape and size, are affected by a physical change while in Chemical changes the substance's physical and chemical qualities, as well as its makeup. A physical change includes little or no heat transfer, but absorption and development of energy occur during a chemical process.
The example of physical change and chemical change are
Chemical change: rusting of iron.
Physical change: melting of ice.