Difference Between Polar and Non Polar
Have you ever wondered why water easily dissolves salt, while oil refuses to mix with it? Why do some molecules attract each other, whereas others show no interaction? The answer lies in the difference between polar and non-polar molecules. Polarity is described as the uneven distribution of electrons in a molecule, which is taken into account because of the electronegativity of the atom.
This Story also Contains
- Polar And Non-polar Substances
- Polar Substances
- Non-Polar Substances
- Difference Between Polar And Non-Polar Molecules
- What Is Polar and Non-polar Bond?
- Difference Between Polar And Non-polar Bonds
- Difference Between Polar And Non-Polar Solvents
- The Boiling Point Of Polar And Nonpolar Molecules
- Some Solved Examples
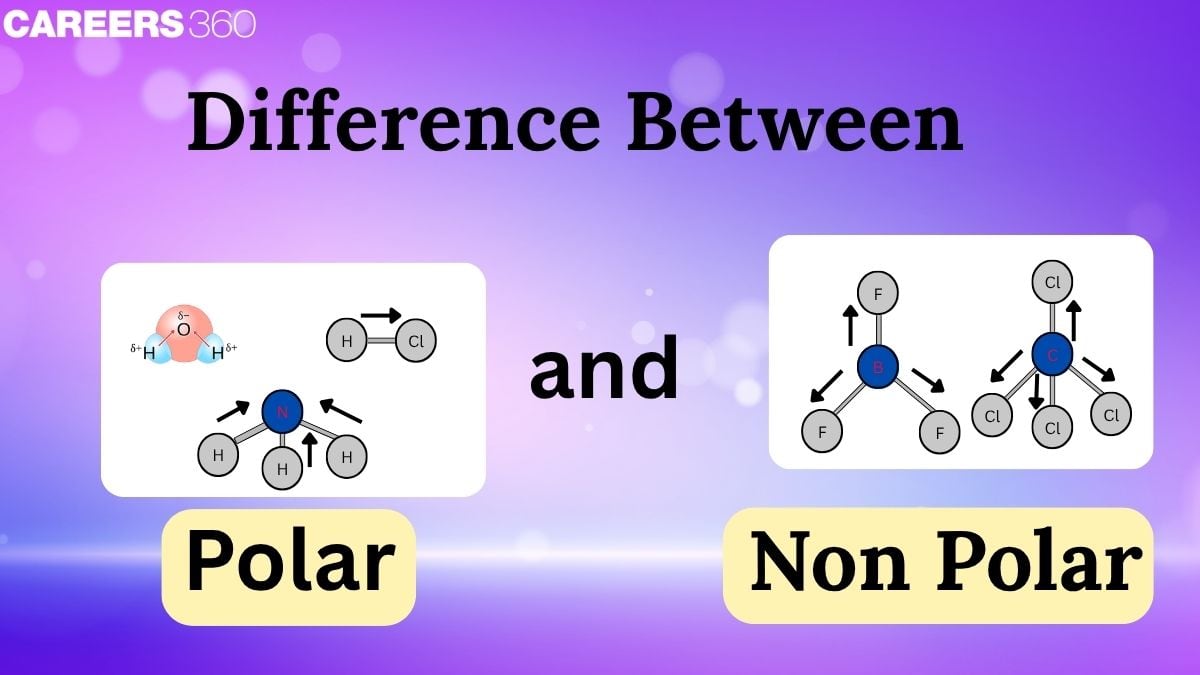
The polarity of a bond is decided mainly by electronegativity. The higher the difference between the electronegativity of atoms forming a bond, the higher the polarity of that bond. On the basis of the polarity of bonds, we can categorize the compounds mainly into two categories, i.e, Polar compounds and Nonpolar compounds. The solubility of compounds is determined by the polarity of the solute and the solvent. Polarity also describes the symmetric and asymmetric nature of compounds. In this article, we will study the nature, types, and properties of various kinds of bonds based on bond polarity.
Polar And Non-polar Substances
What is Polarity?
"A state or a condition of an atom or a molecule having positive and also negative charges, especially in the case of a magnetic or an electrical pole."
How to Distinguish Between Polar and Nonpolar Molecules?
One can predict if a molecule is polar or nonpolar by observing the type of chemical bonds are formed in between the atoms of an elements. If there is a significant difference in between the electronegativity of the bonded atoms, the electron cloud won't be shared equally throughout the bond formed by the atoms. The atom that is more prone towards electrons will have a partial negative charge, while the atom that is less electronegative (i.e., more electropositive) will have a partial positive charge.
Polarity can be simply predicted by considering the Lewis dot structure of the molecule. If the dipole moments (due to the electronegativity of an atom) of a molecule cancel each other out, the molecule is considered to be nonpolar, whereas If the dipole moments don't cancel out each other, the molecule is considered to be polar. Every molecule doesn't possess a dipole moment. For example, a molecule that has a mirror plane won't possess a dipole moment as the individual dipole moments can't lie in more than one dimension.
Polar Substances
A polar molecule is a molecule when one end of the molecule is partially positive, while the other end is partially negative. A diatomic molecule which contains a polar covalent bond in between two hetero atoms, such as HF, is considered to be a polar molecule. The two electrically charged regions (positive and negative) on both ends of the bond of the molecule are called poles. A molecule containing two such poles is termed a dipole. Hydrogen fluoride is an example of a dipole. The formation of dipole results from an unequal distribution of electron cloud density throughout the bond between two atoms of the molecule.
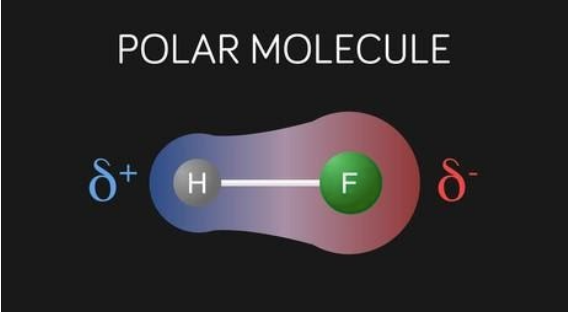
The Polar molecules, usually, orient themselves in the presence of an electric field, such that, the positive ends of the molecules are attracted to the negative plate whereas, the negative ends of the molecules are attracted to the positive plate.
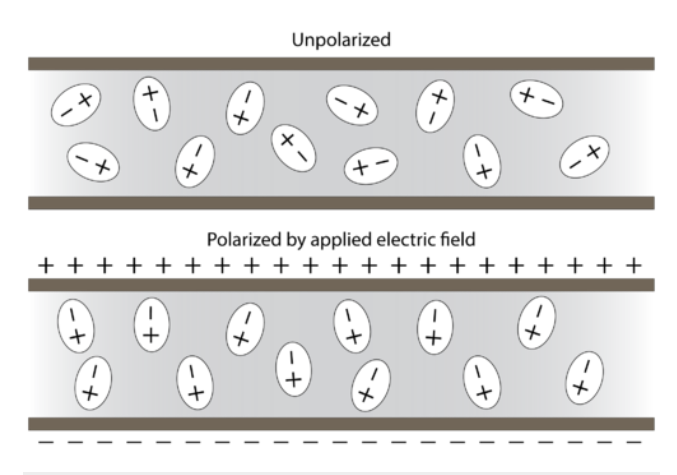
In the absence of an applied electric field Polar molecules remain randomly oriented. In the presence of an electric field, the molecules orient themselves because of the attraction of opposite charges.
Non-Polar Substances
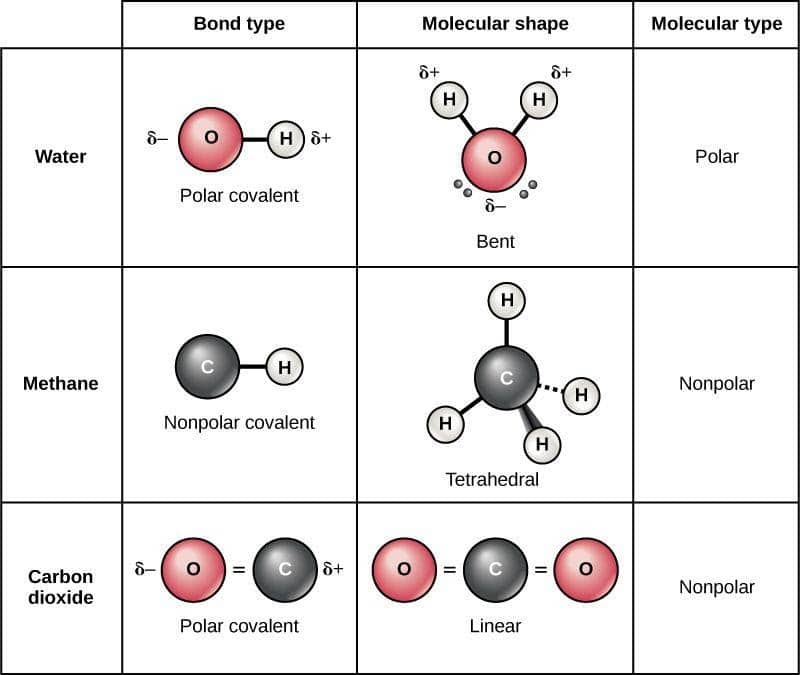
A molecule in which electrons are magnificently distributed does not have the charge difference present at the end. As a result, the symmetrically distributed charges cancel each other out. They are called the non-polar molecules. A solution of a polar molecule cannot be mixed with a non-polar molecule ("like dissolve like"). For example, water and oil. Here, water is referred to as a polar molecule, and oil is referred to as a nonpolar molecule. Water and oil are immiscible and thus do not form solutions.
Nonpolar molecules are symmetric. For example, a tetrahedral molecule such as $\mathrm{CCl}_4$ is nonpolar; boron trifluoride, $\mathrm{BF}_3$ is a trigonal planar molecule and nonpolar.
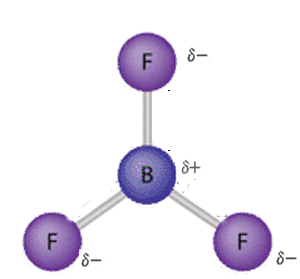
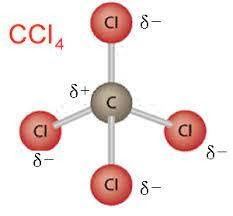
Polar and nonpolar molecules examples (list of polar molecules and non-polar molecules)
A molecule may be considered as polar or nonpolar on the basis of a few criteria. A nonpolar molecule has a linear structure such that the orbital electrons in the outer region can cancel out the net electronegativity.
- In general, pyramid-shaped and V-shaped molecules are considered to be polar. Whereas the Linear molecules are usually non-polar in nature.
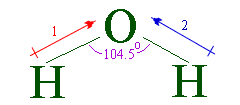
- Water is said to be a polar molecule because of the high electronegativity difference between the oxygen atom and the hydrogen. Oxygen is a highly electronegative atom when compared to hydrogen.
- Examples of non-polar molecules are fats, gasoline, oil, and petrol. They are not soluble in a polar medium like water as nonpolar molecules are insoluble in water.
Polar molecule examples
- Water is a polar molecule. The bonds between hydrogen and oxygen are distributed as if the hydrogen atoms are both on each side of the oxygen atom rather than evenly placed. The oxygen contains a partial negative whereas hydrogen contains a partial positive charge.
- Ethanol is a polar molecule. The oxygen atoms of the hydroxyl group attract electrons because they are more highly electronegative than other atoms in the molecule. Thus the hydroxyl group in ethanol has a partially negative charge.
- Ammonia $\left(\mathrm{NH}_3\right)$ is a polar molecule.
- Sulfur dioxide $\left(\mathrm{SO}_2\right)$ is a polar molecule.
- Hydrogen sulfide $\left(\mathrm{H}_2 \mathrm{~S}\right)$ is a polar molecule.
In carbon dioxide, polar bonds are present but the dipole moments cancel out each other due to its linear shape. Hence, it is a nonpolar molecule.
Examples of nonpolar molecules
Few examples of non-polar molecules are oxygen, ozone, nitrogen, carbon dioxide, methane, gasoline,etc. Examples of homonuclear nonpolar molecules are oxygen $\left(\mathrm{O}_2\right)$, nitrogen $\left(\mathrm{N}_2\right)$, and ozone $\left(\mathrm{O}_3\right)$.
Alkynes are other examples of nonpolar molecules as they are insoluble in water.
The noble gases are also non-polar in nature.
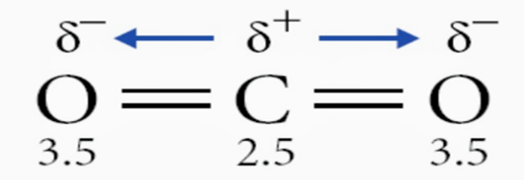
Difference Between Polar And Non-Polar Molecules
|
Polar Molecules
|
Non-Polar Molecules
|
|
Polar molecular forces are strong forces that form H-bonds or dipole-dipole bonds.
| Non-polar molecular forces are the weakest forces that form London dispersed forces. |
| Polar molecules have a net dipole. | Non-polar molecules do not have a net dipole. |
| The difference in the electronegativity between atoms is less than 0.4 | The difference in the electronegativity between atoms is greater than 0.4 |
| Polar molecules have a high boiling point and a high melting point. | Non-polar molecules have a low boiling point and a low melting point. |
| Polar molecules have a low vapor pressure. | Non-polar molecules have a high vapor pressure. |
| Polar molecules have high surface tension. | Non-polar molecules have low surface tension. |
| Polar molecules are asymmetrical and contain either lone pairs of electrons around the central atom. | Nonpolar molecules are symmetrical with no unshared electrons. |
| In polar covalent molecules, one or more than one polar covalent bond is present. | In all non-polar molecules, it is not necessary that a nonpolar covalent bond is present. |
| Examples: Water, HF, $\mathrm{CHCl}_3$ | Examples: Pentane, Hexane, Carbon Dioxide |
What Is Polar and Non-polar Bond?
Nonpolar Covalent Bonds
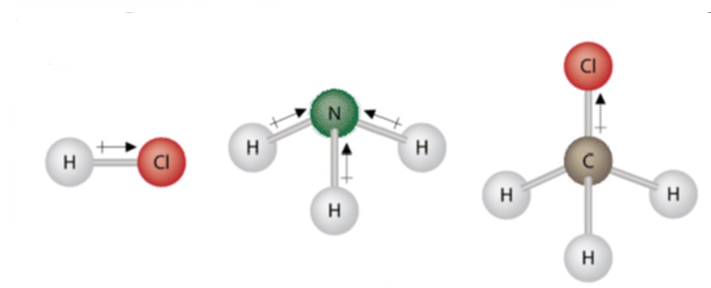
In covalent bonds, the electronegativity difference is less than 1.7. In a nonpolar covalent bond bonding electrons are shared equally among the two electrons.
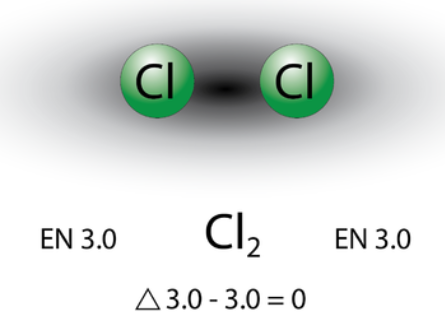
In a chlorine molecule, the two chlorine atoms share an electron pair in a single covalent bond and the chlorine molecule is symmetrical as it is surrounded by electron density. In a molecule when the electronegativity difference is less than 0.4 is referred to as a nonpolar covalent bond. Example: bonding between chlorine and bromine.
$(\Delta E N=3.0-2.8=0.2)$
Polar covalent bonds
In polar covalent bond electronegativity, the difference between the atoms varies between 0.4 and 1.7.
In polar covalent bonds unequal attraction of electrons is seen. The distribution of electrons surrounding the molecule is not symmetrical.
In polar covalent bond, the Greek letter delta ( $\delta$ ) is used to show uneven electron distribution.
In hydrogen fluoride, the electron density is unevenly distributed. Higher density is towards fluorine and lower density is towards hydrogen atom.
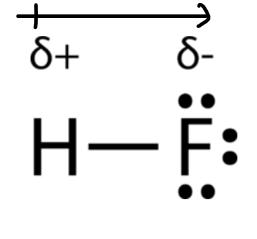
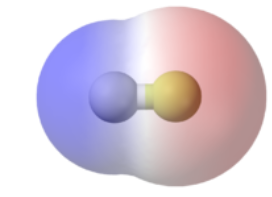
The atom having higher electronegativity acquires a partial negative charge whereas the atom with less electronegativity acquires a partial positive charge.
Difference Between Polar And Non-polar Bonds
|
Polar covalent bonds
|
Nonpolar covalent bonds
|
|
Polar bonds are covalent bonds between two atoms which have different electronegativities
| Polar bonds are covalent bonds between two atoms which have the same electronegativities |
| Electron cloud is distorted throughout the bond | Electron cloud distribution is equal throughout the bond |
| They have charges building up at their poles | They don’t have such charges built up. |
| Polar covalent bonds have dipole moment | It doesn’t have a dipole moment |
| Hydrogen bonding occurs at the charged poles of polar bonds | Van Der Waals force of attraction is usually observed |
Difference Between Polar And Non-Polar Solvents
|
Polar solvents
|
Non-polar solvents
| |
|
Definition
| Those solvents that possess high dipole moment are polar solvents | These solvents don’t possess a dipole moment |
| Ability to dissolve compounds | Dissolve polar compounds | Dissolve non-polar compounds |
| Charge separation | Partial positive charges and partial negative charges are observed | No charge separation |
| Example | Water, methanol, isopropanol, etc. | Chloroform, toluene, hexane etc. |
The Boiling Point Of Polar And Nonpolar Molecules
The existence of dipole forces explains why polar molecules have higher boiling points and melting points than nonpolar molecules. In the following table, we compare the boiling points of several pairs of molecules. In each pair, one molecule is polar and the other is nonpolar, but otherwise, they are as similar as possible. The polar substance always has a higher boiling point, indicating greater attractive forces between separate molecules, that is, larger intermolecular forces.
|
Molecule |
Type |
Boiling Point (°C) |
Reason for boiling point | |
|
Water (H₂O) |
Polar |
100 |
Strong hydrogen bonding (highest boiling point in group). | |
|
Ammonia (NH₃) |
Polar |
-33.3 |
| |
|
Ethanol (C₂H₅OH) |
Polar |
78.4 |
Hydrogen bonding (O-H group). | |
|
Acetone (CH₃COCH₃) |
Polar |
56 |
Dipole-dipole interactions (no H-bonding). | |
|
Methane (CH₄) |
Nonpolar |
-161.5 |
Only London dispersion forces (weakest interactions). | |
|
Hexane (C₆H₁₄) |
Nonpolar |
69 |
London forces (larger molecule = stronger dispersion) | |
|
Carbon Tetrachloride (CCl₄) |
Nonpolar |
76.7 |
London forces (despite polar bonds, symmetry makes it nonpolar) | |
|
Oxygen (O₂) |
Nonpolar |
-183 |
Very weak London forces (small, nonpolar molecule). |
Also Read:
Some Solved Examples
Example 1: Displacement of $\pi$ electron of a multiple bond towards the atom or away from the atom at the demand of reagent is called
1) (correct) Electromeric effect
2) inductive effect
3) mesomeric effect
4) hyperconjugation
Solution
As we have learned
The displacement of $\pi$ electrons in multiple bonds towards the atom or away from the atom at the demand of a reagent is called the electromeric effect.
Hence, the answer is the option (1).
Example 2: Given below are two statements:
Statement I: $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$ and AgCN both can generate nucleophile.
Statement II: KCN and AgCN both will generate nitrile nucleophiles with all reaction conditions.
Choose the most appropriate option:
1) Both Statement I and Statement II are true
2) Both Statement I and Statement II are false
3) (correct) Statement I is true but Statement II is false
4) Statement I is false but Statement II is true
Solution
We know the below facts -
$\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$ and AgCN both can generate nucleophiles.
KCN and AgCN both will NOT generate nitrile nucleophiles with all reaction conditions.
So, Statement I is true but Statement II is false.
Hence, the answer is the option (3).
Example 3: The strongest acid amongst the following compounds is :
1) $\mathrm{CH}_3 \mathrm{COOH}$
2) HCOOH
3) (correct) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}(\mathrm{Cl}) \mathrm{CO}_2 \mathrm{H}$
4) $\mathrm{ClCH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{COOH}$
Solution
$
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}(\mathrm{Cl}) \mathrm{CO}_2 \mathrm{H}
$
$\alpha-$ chlorobutyric acid is a stronger acid than others due to the -e effect (electron-withdrawing group) of Cl.
Hence, the answer is the option (3).
Example 4: Select an incorrect statement about the Electromeric effect
1) It involves polarisation of $\pi$ electrons in the presence of a reagent
2) It is a temporary effect
3) Electrophilic reagents are generally the cause for this effect in Alkenes
4) (correct) The polarization of $\pi$ electrons persists even after the reagent has been removed from the system
Solution
The electromagnetic effect is a temporary effect in which the $\pi$ electrons are polarised in the presence of an attacking reagent.
The polarization is not present in the absence of the reagent
Hence, the answer is option(4).
Practice more question with the link given below
Also read
Frequently Asked Questions (FAQs)
Electronegativity is the prime factor in determining bond nature
Yes, Polars are good conductors of electricity
Water $\left(\mathrm{H}_2 \mathrm{O}\right)$, Ammonia $\left(\mathrm{NH}_3\right)$, Hydrogen Sulphide $\left(\mathrm{H}_2 \mathrm{~S}\right)$
Fats, Gasoline, Petrol, Oil etc
Polarity can be defined as "A state or a condition of an atom or a molecule having positive and also negative charges, especially in case of magnetic or an electrical pole." Water $\left(\mathrm{H}_2 \mathrm{O}\right)$ is an example of polar molecules.
Electronegativity is the prime factor to determine bond nature
Yes, Polars are good conductors of electricity
Water $\left(\mathrm{H}_2 \mathrm{O}\right)$, Ammonia $\left(\mathrm{NH}_3\right)$, Hydrogen Sulphide $\left(\mathrm{H}_2 \mathrm{~S}\right)$
Fats, Gasoline, Petrol, Oil etc
Polarity can be defined as "A state or a condition of an atom or a molecule having positive and also negative charges, especially in case of magnetic or an electrical pole." Water $\left(\mathrm{H}_2 \mathrm{O}\right)$ is an example of polar molecules.
